Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy
- PMID: 29461977
- PMCID: PMC5873857
- DOI: 10.1172/JCI98589
Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy
Erratum in
-
Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy.J Clin Invest. 2018 Nov 1;128(11):5185. doi: 10.1172/JCI124649. Epub 2018 Nov 1. J Clin Invest. 2018. PMID: 30382944 Free PMC article. No abstract available.
Abstract
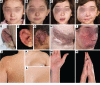
Background: Sporadic vascular malformations (VMs) are complex congenital anomalies of blood vessels that lead to stroke, life-threatening bleeds, disfigurement, overgrowth, and/or pain. Therapeutic options are severely limited, and multidisciplinary management remains challenging, particularly for high-flow arteriovenous malformations (AVM).
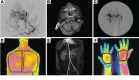
Methods: To investigate the pathogenesis of sporadic intracranial and extracranial VMs in 160 children in which known genetic causes had been excluded, we sequenced DNA from affected tissue and optimized analysis for detection of low mutant allele frequency.
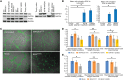
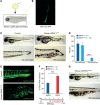
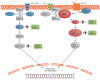
Results: We discovered multiple mosaic-activating variants in 4 genes of the RAS/MAPK pathway, KRAS, NRAS, BRAF, and MAP2K1, a pathway commonly activated in cancer and responsible for the germline RAS-opathies. These variants were more frequent in high-flow than low-flow VMs. In vitro characterization and 2 transgenic zebrafish AVM models that recapitulated the human phenotype validated the pathogenesis of the mutant alleles. Importantly, treatment of AVM-BRAF mutant zebrafish with the BRAF inhibitor vemurafinib restored blood flow in AVM.
Conclusion: Our findings uncover a major cause of sporadic VMs of different clinical types and thereby offer the potential of personalized medical treatment by repurposing existing licensed cancer therapies.
Funding: This work was funded or supported by grants from the AVM Butterfly Charity, the Wellcome Trust (UK), the Medical Research Council (UK), the UK National Institute for Health Research, the L'Oreal-Melanoma Research Alliance, the European Research Council, and the National Human Genome Research Institute (US).
Keywords: Drug therapy; Molecular genetics; Signal transduction; Therapeutics; Vascular Biology.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

