A mouse model study of toxicity and biodistribution of a replication defective adenovirus serotype 5 virus with its genome engineered to contain a decoy hyper binding site to sequester and suppress oncogenic HMGA1 as a new cancer treatment therapy
- PMID: 29462157
- PMCID: PMC5819794
- DOI: 10.1371/journal.pone.0192882
A mouse model study of toxicity and biodistribution of a replication defective adenovirus serotype 5 virus with its genome engineered to contain a decoy hyper binding site to sequester and suppress oncogenic HMGA1 as a new cancer treatment therapy
Abstract
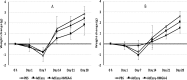
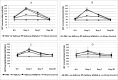
The HGMA1 architectural transcription factor is highly overexpressed in many human cancers. Because HMGA1 is a hub for regulation of many oncogenes, its overexpression in cancer plays a central role in cancer progression and therefore HMGA1 is gaining increasing attention as a target for development of therapeutic approaches to suppress either its expression or action in cancer cells. We have developed the strategy of introducing decoy hyper binding sites for HMGA1 into the nucleus of cancer cells with the goal of competetively sequestering overexpressed HMGA1 and thus suppressing its oncogenic action. Towards achieving this goal, we have introduced an HMGA1 decoy hyper binding site composed of six copies of a high affinity HMGA1 binding site into the genome of the replication defective adenovirus serotype 5 genome and shown that the engineered virus effectively reduces the viability of human pancreatic and cancer cells. Here we report the first pre-clinical measures of toxicity and biodistribution of the engineered virus in C57BL/6J Black 6 mice. The immune response to exposure of the engineered virus was determined by assaying the serum levels of key cytokines, IL-6 and TNF-α. Toxicity due to exposure to the virus was determined by measuring the serum levels of the liver enzymes aspartate aminotransferase and alanine aminotransferase. Biodistribution was measured following direct injection into the pancreas or liver by quantifying viral loads in the pancreas, liver, spleen and brain.
Conflict of interest statement
Figures


References
-
- Pearson S, Jia H, Kandachi K. China approves first gene therapy. Nat Biotechnol. 2004; 22: 3–4. doi: 10.1038/nbt0104-3 - DOI - PMC - PubMed
-
- Benihoud K, Yeh P, Perricaudet M. Adenovirus vectors for gene delivery. Curr Opin Biotechnol. 1999; 10: 440–7. - PubMed
-
- Huang D, Pereboev A, Korokhov N, He R, Larocque L, Gravel C, et al. Significant alterations of biodistribution and immune responses in Balb/c mice administered with adenovirus targeted to CD40(+) cells. Gene Ther. 2008; 15: 298–308. doi: 10.1038/sj.gt.3303085 - DOI - PMC - PubMed
-
- Crosby CM, Barry MA. Transgene Expression and Host Cell Responses to Replication-Defective, Single-Cycle, and Replication-Competent Adenovirus Vectors. Genes. 2017; 8 doi: 10.3390/genes8020079 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

