SNP markers tightly linked to root knot nematode resistance in grapevine (Vitis cinerea) identified by a genotyping-by-sequencing approach followed by Sequenom MassARRAY validation
- PMID: 29462210
- PMCID: PMC5819801
- DOI: 10.1371/journal.pone.0193121
SNP markers tightly linked to root knot nematode resistance in grapevine (Vitis cinerea) identified by a genotyping-by-sequencing approach followed by Sequenom MassARRAY validation
Abstract
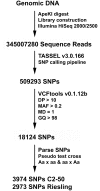
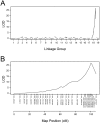
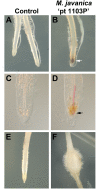
Plant parasitic nematodes, including root knot nematode Meloidogyne species, cause extensive damage to agriculture and horticultural crops. As Vitis vinifera cultivars are susceptible to root knot nematode parasitism, rootstocks resistant to these soil pests provide a sustainable approach to maintain grapevine production. Currently, most of the commercially available root knot nematode resistant rootstocks are highly vigorous and take up excess potassium, which reduces wine quality. As a result, there is a pressing need to breed new root knot nematode resistant rootstocks, which have no impact on wine quality. To develop molecular markers that predict root knot nematode resistance for marker assisted breeding, a genetic approach was employed to identify a root knot nematode resistance locus in grapevine. To this end, a Meloidogyne javanica resistant Vitis cinerea accession was crossed to a susceptible Vitis vinifera cultivar Riesling and results from screening the F1 individuals support a model that root knot nematode resistance, is conferred by a single dominant allele, referred as MELOIDOGYNE JAVANICA RESISTANCE1 (MJR1). Further, MJR1 resistance appears to be mediated by a hypersensitive response that occurs in the root apical meristem. Single nucleotide polymorphisms (SNPs) were identified using genotyping-by-sequencing and results from association and genetic mapping identified the MJR1 locus, which is located on chromosome 18 in the Vitis cinerea accession. Validation of the SNPs linked to the MJR1 locus using a Sequenom MassARRAY platform found that only 50% could be validated. The validated SNPs that flank and co-segregate with the MJR1 locus can be used for marker-assisted selection for Meloidogyne javanica resistance in grapevine.
Conflict of interest statement
Figures



References
-
- Davies LJ, Elling AA (2015) Resistance genes against plant-parasitic nematodes: a durable control strategy? Nematology 17: 249–263.
-
- Saucet SB, Van Ghelder C, Abad P, Duval H, Esmenjaud D (2016) Resistance to root-knot nematodes Meloidogyne spp. in woody plants. New Phytologist 211: 41–56. doi: 10.1111/nph.13933 - DOI - PubMed
-
- Palomares-Rius JE, Escobar C, Cabrera J, Vovlas A, Castillo P (2017) Anatomical Alterations in Plant Tissues Induced by Plant-Parasitic Nematodes. Frontiers in Plant Science 8: 1987 doi: 10.3389/fpls.2017.01987 - DOI - PMC - PubMed
-
- Nicol JM, Stirling GR, Rose BJ, May P, Van Heeswijck R (1999) Impact of nematodes on grapevine growth and productivity: current knowledge and future directions, with special reference to Australian viticulture. Australian Journal of Grape and Wine Research 5: 109–107.
-
- Williamson VM, Gleason CA (2003) Plant-nematode interactions. Current Opinion in Plant Biology 6: 327–333. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

