Mechanisms for the establishment and maintenance of pregnancy: synergies from scientific collaborations
- PMID: 29462279
- PMCID: PMC6044348
- DOI: 10.1093/biolre/ioy047
Mechanisms for the establishment and maintenance of pregnancy: synergies from scientific collaborations
Abstract
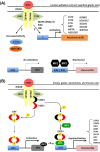
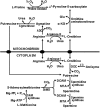
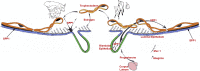
Research on the functions of interferon tau (IFNT) led to the theory of pregnancy recognition signaling in ruminant species. But IFNT does much more as it induces expression of interferon regulatory factor 2 (IRF2) in uterine luminal (LE), superficial glandular (sGE), but not glandular (GE) epithelia. First, IRF2 silences transcription of the estrogen receptor alpha gene and, indirectly, transcription of the oxytocin receptor gene to abrogate development of the luteolytic mechanism to prevent regression of the corpus luteum and its production of progesterone for establishing and maintaining pregnancy. Second, IRF2 silences expression of classical interferon-stimulated genes in uterine LE and sGE; however, uterine LE and sGE respond to progesterone (P4) and IFNT to increase expression of genes for transport of nutrients into the uterine lumen such as amino acids and glucose. Other genes expressed by uterine LE and sGE encode for adhesion molecules such as galectin 15, cathepsins, and cystatins for tissue remodeling, and hypoxia-inducible factor relevant to angiogenesis and survival of blastocysts in a hypoxic environment. IFNT is also key to a servomechanism that allows uterine epithelia, particularly GE, to proliferate and to express genes in response to placental lactogen and placental growth hormone in sheep. The roles of secreted phosphoprotein 1 are also discussed regarding its role in implantation in sheep and pigs, as well as its stimulation of expression of mechanistic target of rapamycin mRNA and protein which is central to proliferation, migration, and gene expression in the trophectoderm cells.
Figures
References
-
- Schramm W, Bovaird L, Glew ME, Schramm G, McCracken JA. Corpus luteum regression induced by ultra-low pulses of prostaglandin F2 alpha. Prostaglandins 1983; 26:347–364. - PubMed
-
- Ott TL, Zhou Y, Mirando MA, Stevens C, Harney JP, Ogle TF, Bazer FW. Changes in progesterone and oestrogen receptor mRNA and protein during maternal recognition of pregnancy and luteolysis in ewes. J Mol Endocrinol 1993; 10:171–183. - PubMed
-
- Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod 1995; 53:1527–1543. - PubMed
-
- Fincher KB, Bazer FW, Hansen PJ, Thatcher WW, Roberts RM. Proteins secreted by the sheep conceptus suppress induction of uterine prostaglandin F2a release by oestradiol and oxytocin. Reproduction 1986; 76:425–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

