Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis
- PMID: 29462348
- PMCID: PMC6322443
- DOI: 10.1093/ndt/gfx378
Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis
Erratum in
-
Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis.Nephrol Dial Transplant. 2018 May 1;33(5):899. doi: 10.1093/ndt/gfy075. Nephrol Dial Transplant. 2018. PMID: 29617842 Free PMC article. No abstract available.
Abstract
Background: Current guidelines advise that rituximab or cyclophosphamide should be used for the treatment of organ-threatening disease in anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV), although few studies have examined the efficacy and safety of these agents in combination.
Methods: We conducted a single-centre cohort study of 66 patients treated with a combination of oral corticosteroids, rituximab and low-dose pulsed intravenous cyclophosphamide followed by a maintenance regimen of azathioprine and tapered steroid for the treatment of biopsy-proven renal involvement in AAV. Patients were followed for a median of 56 months. Case-control analysis with 198 propensity-matched cases from European Vasculitis Study Group (EUVAS) trials compared long-term differences in relapse-free, renal and patient survival.
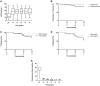
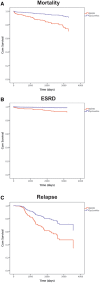
Results: At entry, the median Birmingham Vasculitis Activity Score (BVAS) was 19 and estimated glomerular filtration rate was 25 mL/min. Cumulative doses of rituximab, cyclophosphamide and corticosteroids were 2, 3 and 4.2 g, respectively, at 6 months. A total of 94% of patients achieved disease remission by 6 months (BVAS < 0) and patient and renal survival were 84 and 95%, respectively, at 5 years. A total of 84% achieved ANCA-negative status and 57% remained B cell deplete at 2 years, which was associated with low rates of major relapse (15% at 5 years). The serious infection rate during long-term follow-up was 1.24 per 10 patient-years. Treatment with this regimen was associated with a reduced risk of death {hazard ratio [HR] 0.29 [95% confidence interval (CI) 0.125-0.675], P = 0.004}, progression to end-stage renal disease (ESRD) [HR 0.20 (95% CI 0.06-0.65), P = 0.007] and relapse [HR 0.49 (95% CI 0.25-0.97), P = 0.04] compared with propensity-matched patients enrolled in EUVAS trials.
Conclusions: This regimen is potentially superior to current standards of care, and controlled studies are warranted to establish the utility of combination drug approaches in the treatment of AAV.
Figures


References
-
- Keogh KA, Wylam ME, Stone JH. et al. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52: 262–268 - PubMed
-
- Jones RB, Ferraro AJ, Chaudhry AN. et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2009; 60: 2156–2168 - PubMed
-
- Jones RB, Tervaert JW, Hauser T. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–220 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

