Stochastic Pharmacokinetic-Pharmacodynamic Modeling for Assessing the Systemic Health Risk of Perfluorooctanoate (PFOA)
- PMID: 29462473
- PMCID: PMC5920327
- DOI: 10.1093/toxsci/kfy035
Stochastic Pharmacokinetic-Pharmacodynamic Modeling for Assessing the Systemic Health Risk of Perfluorooctanoate (PFOA)
Abstract
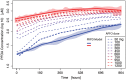
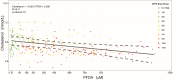
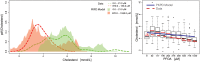
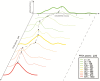
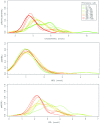
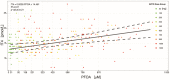
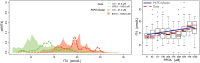
A phase 1 dose-escalation trial assessed the chemotherapeutic potential of ammonium perfluorooctanoate (APFO). Forty-nine primarily solid-tumor cancer patients who failed standard therapy received weekly APFO doses (50-1200 mg) for 6 weeks. Clinical chemistries and plasma PFOA (anionic APFO) were measured predose and weekly thereafter. Several clinical measures including total cholesterol, high-density lipoproteins (HDLs), thyroid stimulating hormone (TSH), and free thyroxine (fT4), relative to PFOA concentrations were examined by: Standard statistical analyses using generalized estimating equations (GEE) and a probabilistic analysis using probability distribution functions (pdf) at various PFOA concentrations; and a 2-compartment pharmacokinetic/pharmacodynamic (PK/PD) model to directly estimate mean changes. Based on the GEE, the average rates of change in total cholesterol and fT4 associated with increasing PFOA were approximately -1.2×10-3 mmol/l/μM and 2.8×10-3 pmol/l/μM, respectively. The PK/PD model predicted more closely the trends observed in the data as well as the pdfs of biomarkers. A decline in total cholesterol was observed, with a clear transition in shape and range of the pdfs, manifested by the maximum value of the Kullback-Leibler (KL) divergence, that occurred at plasma PFOA between 420 and 565 μM (175 000-230 000 ng/ml). High-density lipoprotein was unchanged. An increase in fT4 was observed at a higher PFOA transition point, albeit TSH was unchanged. Our findings are consistent with some animal models and may motivate re-examination of the epidemiologic studies to PFOA at levels several orders of magnitude lower than this study. These observational studies have reported contrary associations, but currently understood biology does not support the existence of such conflicting effects.
Figures


References
-
- Albrecht P. P., Torsell N. E., Krishnan P., Ehresman D. J., Frame S. R., Chang S. C., Butenhoff J. L., Kennedy G. L., Gonzalez F. J., Peters J. M. (2013). A species difference in the perixosome proliferator-activated receptor α-dependent response to the developmental effects of perfluorooctanoic acid. Toxicol. Sci. 131, 568–582. - PubMed
-
- Barnett A. D., Ding S., Murray C., Chamberlain M., Plummer S., Evans T. R. J., MacPherson I., Bissett D., Elcombe C. R., Wolf C. R. (2010). Anti-tumor activity of CXR 1002, a novel anti-cancer clinical phase compound that induces ER stress and inhibits PIM kinases: Human tumor xenograft efficacy and in vitro mode of action. (Abstract 123). EJC Suppl 8, 45–46.
-
- Benbrahim-Tallaa L., Lauby-Secretan B., Loomis D., Guyton K. Z., Grosse Y., Ghissassi F. E., Bouvard V., Guha N., Mattock H., Straif K. (2014). Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1, 2-dichloropropane, and 1, 3-propane sultone. Lancet Oncol. 15, 924–925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

