The human T-cell leukemia virus type-1 p30II protein activates p53 and induces the TIGAR and suppresses oncogene-induced oxidative stress during viral carcinogenesis
- PMID: 29462755
- PMCID: PMC5911249
- DOI: 10.1016/j.virol.2018.02.010
The human T-cell leukemia virus type-1 p30II protein activates p53 and induces the TIGAR and suppresses oncogene-induced oxidative stress during viral carcinogenesis
Abstract
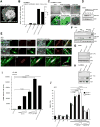
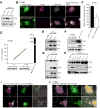
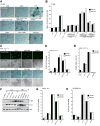
In normal cells, aberrant oncogene expression leads to the accumulation of cytotoxic metabolites, including reactive oxygen species (ROS), which can cause oxidative DNA-damage and apoptosis as an intrinsic barrier against neoplastic disease. The c-Myc oncoprotein is overexpressed in many lymphoid cancers due to c-myc gene amplification and/or 8q24 chromosomal translocations. Intriguingly, p53 is a downstream target of c-Myc and hematological malignancies, such as adult T-cell leukemia/lymphoma (ATL), frequently contain wildtype p53 and c-Myc overexpression. We therefore hypothesized that p53-regulated pro-survival signals may thwart the cell's metabolic anticancer defenses to support oncogene-activation in lymphoid cancers. Here we show that the Tp53-induced glycolysis and apoptosis regulator (TIGAR) promotes c-myc oncogene-activation by the human T-cell leukemia virus type-1 (HTLV-1) latency-maintenance factor p30II, associated with c-Myc deregulation in ATL clinical isolates. TIGAR prevents the intracellular accumulation of c-Myc-induced ROS and inhibits oncogene-induced cellular senescence in ATL, acute lymphoblastic leukemia, and multiple myeloma cells with elevated c-Myc expression. Our results allude to a pivotal role for p53-regulated antioxidant signals as mediators of c-Myc oncogenic functions in viral and non-viral lymphoid tumors.
Keywords: ATL; HTLV-1; Oncogene; ROS; TIGAR; p53.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- Awasthi S, Sharma A, Wong K, Zhang J, Matlock EF, Rogers L, Motloch P, Takemoto S, Taguchi H, Cole MD, Luscher B, Dittrich O, Tagami H, Nakatani Y, McGee M, Girard AM, Gaughan L, Robson CN, Monnat RJ, Jr, Harrod R. A human T-cell lymphotropic virus type 1 enhancer of Myc transforming potential stabilizes Myc-TIP60 transcriptional interactions. Mol Cell Biol. 2005;25:6178–6198. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

