Responsive Polydiacetylene Vesicles for Biosensing Microorganisms
- PMID: 29462870
- PMCID: PMC5856053
- DOI: 10.3390/s18020599
Responsive Polydiacetylene Vesicles for Biosensing Microorganisms
Abstract
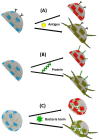
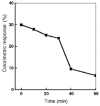
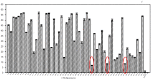
Polydiacetylene (PDA) inserted in films or in vesicles has received increasing attention due to its property to undergo a blue-to-red colorimetric transition along with a change from non-fluorescent to fluorescent upon application of various stimuli. In this review paper, the principle for the detection of various microorganisms (bacteria, directly detected or detected through the emitted toxins or through their DNA, and viruses) and of antibacterial and antiviral peptides based on these responsive PDA vesicles are detailed. The analytical performances obtained, when vesicles are in suspension or immobilized, are given and compared to those of the responsive vesicles mainly based on the vesicle encapsulation method. Many future challenges are then discussed.
Keywords: bacteria; biosensing; peptides; polydiacetylene; toxins; vesicles; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Okada S., Peng S., Spevak W., Charych D. Color and Chromism of Polydiacetylene Vesicles. Acc. Chem. Res. 1998;31:229–239. doi: 10.1021/ar970063v. - DOI
-
- Fendler J.H. Surfactant vesicles as membrane mimetic agents: Characterization and utilization. Acc. Chem. Res. 1980;13:7–13. doi: 10.1021/ar50145a002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

