Glutamine Synthetase: Localization Dictates Outcome
- PMID: 29463059
- PMCID: PMC5852604
- DOI: 10.3390/genes9020108
Glutamine Synthetase: Localization Dictates Outcome
Abstract
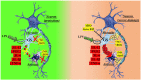
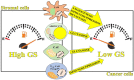
Glutamine synthetase (GS) is the adenosine triphosphate (ATP)-dependent enzyme that catalyses the synthesis of glutamine by condensing ammonium to glutamate. In the circulatory system, glutamine carries ammonia from muscle and brain to the kidney and liver. In brain reduction of GS activity has been suggested as a mechanism mediating neurotoxicity in neurodegenerative disorders. In cancer, the delicate balance between glutamine synthesis and catabolism is a critical event. In vitro evidence, confirmed in vivo in some cases, suggests that reduced GS activity in cancer cells associates with a more invasive and aggressive phenotype. However, GS is known to be highly expressed in cells of the tumor microenvironment, such as fibroblasts, adipocytes and immune cells, and their ability to synthesize glutamine is responsible for the acquisition of protumoral phenotypes. This has opened a new window into the complex scenario of the tumor microenvironment, in which the balance of glutamine consumption versus glutamine synthesis influences cellular function. Since GS expression responds to glutamine starvation, a lower glutamine synthesizing power due to the absence of GS in cancer cells might apply a metabolic pressure on stromal cells. This event might push stroma towards a GS-high/protumoral phenotype. When referred to stromal cells, GS expression might acquire a 'bad' significance to the point that GS inhibition might be considered a conceivable strategy against cancer metastasis.
Keywords: M2 macrophages; adipocytes; brain; cancer; glutaminase; glutamine; glutamine synthetase; immunometabolism; immunosuppressive; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Schreier H.J. Biosynthesis of Glutamine and Glutamate and the Assimilation of Ammonia. In: Sonenshein A., Hoch J., Losick R., editors. Bacillus subtilis and Other Gram-Positive Bacteria. ASM Press; Washington, DC, USA: 1993. pp. 281–298.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

