NEET Proteins: A New Link Between Iron Metabolism, Reactive Oxygen Species, and Cancer
- PMID: 29463105
- PMCID: PMC10625470
- DOI: 10.1089/ars.2018.7502
NEET Proteins: A New Link Between Iron Metabolism, Reactive Oxygen Species, and Cancer
Abstract
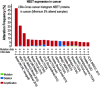
Significance: Cancer cells accumulate high levels of iron and reactive oxygen species (ROS) to promote their high metabolic activity and proliferation rate. However, high levels of iron and ROS can also lead to enhanced oxidative stress and the activation of cell death pathways such as apoptosis and ferroptosis. This has led to the proposal that different drugs that target iron and/or ROS metabolism could be used as anticancer drugs. However, due to the complex role iron and ROS play in cells, the majority of these drugs yielded mixed results, highlighting a critical need to identify new players in the regulation of iron and ROS homeostasis in cancer cells. Recent Advances: NEET proteins belong to a newly discovered class of iron-sulfur proteins (2Fe-2S) required for the regulation of iron and ROS homeostasis in cells. Recent studies revealed that the NEET proteins NAF-1 (CISD2) and mitoNEET (CISD1) play a critical role in promoting the proliferation of cancer cells, supporting tumor growth and metastasis. Moreover, the function of NEET proteins in cancer cells was found to be dependent of the degree of lability of their 2Fe-2S clusters.
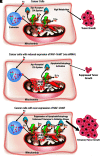
Critical issues: NEET proteins could represent a key regulatory link between the maintenance of high iron and ROS in cancer cells, the activation of cell death and survival pathways, and cellular proliferation.
Future directions: Because the function of NEET proteins depends on the lability of their clusters, drugs that target the 2Fe2S clusters of NEET proteins could be used as promising anticancer drugs.
Keywords: NAF-1; NEET proteins; ROS; cancer; iron–sulfur; mitoNEET.
Figures




References
-
- Bai F, Morcos F, Sohn YS, Darash-Yahana M, Rezende CO, Lipper CH, Paddock ML, Song L, Luo Y, Holt SH, Tamir S, Theodorakis EA, Jennings PA, Onuchic JN, Mittler R, and Nechushtai R. The Fe-S cluster-containing NEET proteins mitoNEET and NAF-1 as chemotherapeutic targets in breast cancer. Proc Natl Acad Sci U S A 112: 3698–3703, 2015. - PMC - PubMed
-
- Benson SK, Boyce KM, Bunker RM, Collins NB, Daily KJ, Esway AS, Gilmore GT, Hartzler CW, Howard GP, Kasmar NA, Kennedy KJ, King BL, Kordahi TN, Mattioli TA, Pugh DM, Ray LA, Ross SL, Torcasio MH, Webber DP, Morris DL, and Leeper TC. Multinuclear NMR and UV–Vis spectroscopy of site directed mutants of the diabetes drug target protein mitoNEET suggest that folding is intimately coupled to iron–sulfur cluster formation. Inorg Chem Commun 63: 86–92, 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

