TEAM-UP for quality: a cluster randomized controlled trial protocol focused on preventing pressure ulcers through repositioning frequency and precipitating factors
- PMID: 29463211
- PMCID: PMC5820803
- DOI: 10.1186/s12877-018-0744-0
TEAM-UP for quality: a cluster randomized controlled trial protocol focused on preventing pressure ulcers through repositioning frequency and precipitating factors
Abstract
Background: Pressure ulcers/injuries (PrUs), a critical concern for nursing homes (NH), are responsible for chronic wounds, amputations, septic infections, and premature deaths. PrUs occur most commonly in older adults and NH residence is a risk factor for their development, with at least one of every nine U.S. NH residents experiencing a PrU and many NHs having high incidence and prevalence rates, in some instances well over 20%. PrU direct treatment costs are greater than prevention costs, making prevention-focused protocols critical. Current PrU prevention protocols recommend repositioning residents at moderate, high, and severe risk every 2 h. The advent of visco-elastic (VE) high-density foam support-surfaces over the past decade may now make it possible to extend the repositioning interval to every 3 or 4 h without increasing PrU development. The TEAM-UP (Turn Everyone And Move for Ulcer Prevention) study aims to determine: 1) whether repositioning interval can be extended for NH residents without compromising PrU incidence and 2) how changes in medical severity interact with changes in risk level and repositioning schedule to predict PrU development.
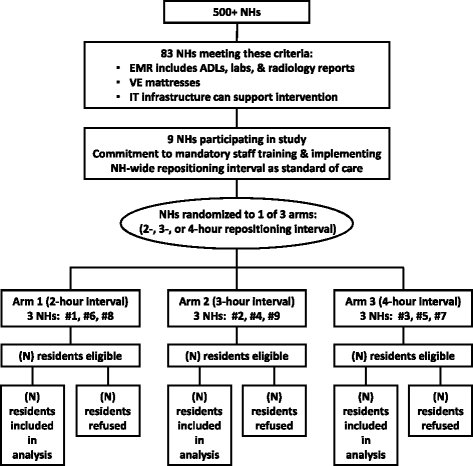
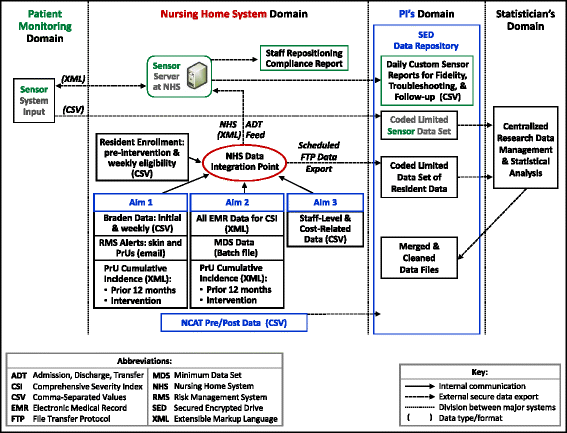
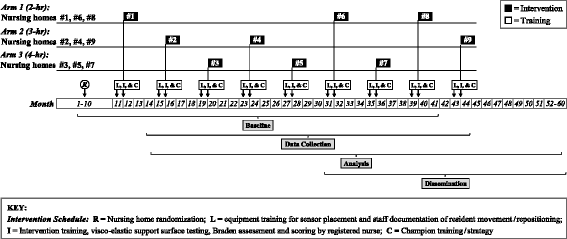
Methods: In this proposed cluster randomized study, 9 NHs will be randomly assigned to one of three repositioning intervals (2, 3, or 4 h) for a 4-week period. Each enrolled site will use a single NH-wide repositioning interval as the standard of care for residents at low, moderate, and high risk of PrU development (N = 951) meeting the following criteria: minimum 3-day stay, without PrUs, no adhesive allergy, and using VE support surfaces (mattresses). An FDA-cleared patient monitoring system that records position/movement of these residents via individual wireless sensors will be used to visually cue staff when residents need repositioning and document compliance with repositioning protocols.
Discussion: This study will advance knowledge about repositioning frequency and clinically assessed PrU risk level in relation to PrU incidence and medical severity. Outcomes of this research will contribute to future guidelines for more precise preventive nursing practices and refinement of PrU prevention guidelines.
Trial registration: Clinical Trial Registration: NCT02996331 .
Keywords: Nursing; Pressure injury; Pressure ulcer; Prevention; Repositioning.
Conflict of interest statement
Ethics approval and consent to participate
The project was approved by Duke University IRB. Waiver of consent was granted because: 1) the entire cluster of low-, moderate-, and high-risk residents will receive a NH-wide repositioning schedule, 2) the repositioning protocol will become part of NH-wide practice standardizing the workflow of repositioning, 3) the cluster-level intervention involves minimal risk, and 4) extracted resident data from PM database, MDS, and EMR will be assigned a study identification (ID) number and coded data set will be placed directly into a secured network folder.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Centers for Medicare & Medicaid Services (CMS) Action plan for (further improvement of) nursing home quality. Baltimore: Centers for Medicare & Medicaid Services; 2008.
-
- Agency for Healthcare Research and Quality (AHRQ) Preventing pressure ulcers in hospitals: a toolkit for improving quality of care. Rockville: Agency for Healthcare Research and Quality; 2011.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous