Prevalence and correlates of non-adherence to immunosuppressants and to health behaviours in patients after kidney transplantation in Brazil - the ADHERE BRAZIL multicentre study: a cross-sectional study protocol
- PMID: 29463231
- PMCID: PMC5819659
- DOI: 10.1186/s12882-018-0840-6
Prevalence and correlates of non-adherence to immunosuppressants and to health behaviours in patients after kidney transplantation in Brazil - the ADHERE BRAZIL multicentre study: a cross-sectional study protocol
Abstract
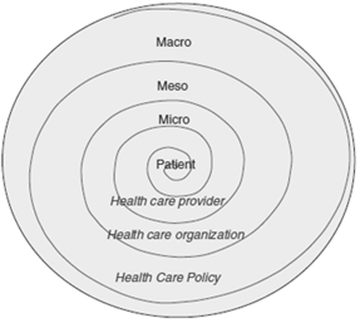
Background: Non-adherence to immunosuppressive therapy is a prevalent risk factor for poor clinical and after kidney transplantation (KT), and has contributed to the lack of improvement in long-term graft survival over the past decade. Understanding the multilevel correlates and risk factors of non-adherence is crucial to determine the optimal level for planning interventions, namely at the patient, health care provider, KT centre, and health care system level. Brazil, having the largest public transplantation program in the world and with regional differences regarding access to health services and service implementation, is in a unique position to study this multilevel approach. Therefore, the Adhere Brazil Study (ADHERE BRAZIL) was designed to assess the prevalence and variability of non-adherence to immunosuppressants and to health behaviours among adult KT recipients in Brazil, and to assess the multilevel correlates of non-adherence to immunosuppressive medication. We describe the rationale, design, and methodology of the ADHERE BRAZIL study.
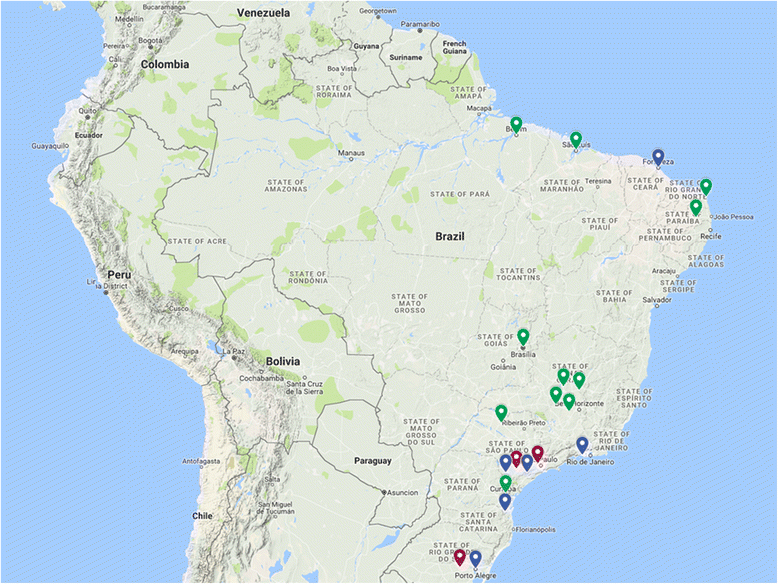
Methods/design: This is an observational, cross-sectional, multicentre study that includes 20 Brazilian KT centres. A stratified sampling approach is used, based on strata, with the following characteristics considered: geographical region and transplant activity (number of KTs per year). A random sample of patients (proportional to the size of the centre within each stratum) is selected from each centre. The prevalence of different health behaviours is assessed through self-report. The assessment of multilevel correlates of non-adherence is guided by the ecological model that considers factors at the level of the patient, health-care professional, and transplant centre, using established instruments or instruments developed for this study. Data will be collected over an 18-month period, with information obtained during the regular follow-up visits to the transplant outpatient clinic and directly entered into the Research Electronic Data Capture (RedCap) system. Data entry is performed by a trained professional who is part of the transplant team. The data collection began in December 2015.
Discussion: This multicentre study is the first to evaluate multilevel correlates of non-adherence in KT patients and will provide a reliable estimate of non-adherence in Brazilian KT patients.
Trial registration: ClinicalTrials.gov on 10/10/2013, NCT02066935 .
Keywords: Brazil; Design; Health behaviour; Healthcare system; Immunosuppression; Kidney transplantation; Medication nonadherence; Patient adherence.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics in Research Committee of the University Hospital of Federal University of Juiz de Fora (approval number – 691.120/2014), which was the coordinating centre and by the local Ethics in Research Committees of all centres involved in the study. After invitation, all patients willing to participate in the study signed a written informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Meier-Kriesche HU, Ojo AO, Port FK, Arndorfer JA, Cibrik DM, Kaplan B. Survival improvement among patients with end-stage renal disease: trends over time for transplant recipients and wait-listed patients. J Am Soc Nephrol. 2001;12(6):1293–1296. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

