Continued in vitro cefazolin susceptibility in methicillin-susceptible Staphylococcus aureus
- PMID: 29463249
- PMCID: PMC5819674
- DOI: 10.1186/s12941-018-0257-x
Continued in vitro cefazolin susceptibility in methicillin-susceptible Staphylococcus aureus
Abstract
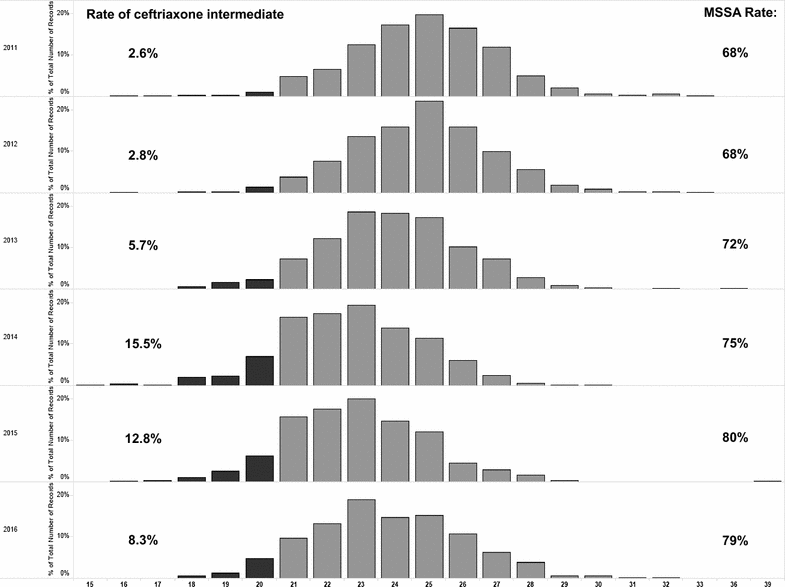
Objectives: In vitro trends of cefazolin and ceftriaxone susceptibilities from pediatric clinical isolates of methicillin-susceptible Staphylococcus aureus (MSSA) between 2011 and 2016 were analyzed for surveillance.
Methods: Our laboratory continues to use agar disk diffusion for staphylococcal susceptibilities applying Clinical Laboratory Standard Institute's 2012 breakpoints.
Results: A total of 3992 MSSA clinical isolates in the last 6 years were analyzed for their in vitro cefazolin and ceftriaxone susceptibilities. While all MSSA isolates exhibited cefazolin susceptibilities within the "susceptible" zone range, there have been a proportion of isolates with ceftriaxone susceptibilities falling in "intermediate" zones, ranging from 2.6% in 2011 to 8.3% in 2016.
Conclusions: Cefazolin continues to be the recommended agent for MSSA treatment at our institution, reflected by the finding that only 2% (6/321) of patients who received ceftriaxone as definitive therapy for MSSA bacteremia during the study period. We have confirmed the cefoxitin-predicted MSSA susceptibility to cefazolin, but have found concerning drifts in ceftriaxone susceptibilities by continued in vitro monitoring over the last 6 years.
Keywords: Cefazolin; Ceftriaxone; MIC; MSSA; Staphylococcus aureus.
Figures

References
-
- Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK, Vancomycin-Resistant Staphylococcus aureus Investigative T Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. - DOI - PubMed
-
- CLSI . Performance standards for antimicrobial susceptibility testing. M100-S22. Wayne: Institute CaLS, Clinical and Laboratory Standards Institute; 2012.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

