Leukocyte telomere length in paediatric critical illness: effect of early parenteral nutrition
- PMID: 29463275
- PMCID: PMC5820800
- DOI: 10.1186/s13054-018-1972-6
Leukocyte telomere length in paediatric critical illness: effect of early parenteral nutrition
Abstract
Background: Children who have suffered from critical illnesses that required treatment in a paediatric intensive care unit (PICU) have long-term physical and neurodevelopmental impairments. The mechanisms underlying this legacy remain largely unknown. In patients suffering from chronic diseases hallmarked by inflammation and oxidative stress, poor long-term outcome has been associated with shorter telomeres. Shortened telomeres have also been reported to result from excessive food consumption and/or unhealthy nutrition. We investigated whether critically ill children admitted to the PICU have shorter-than-normal telomeres, and whether early parenteral nutrition (PN) independently affects telomere length when adjusting for known determinants of telomere length.
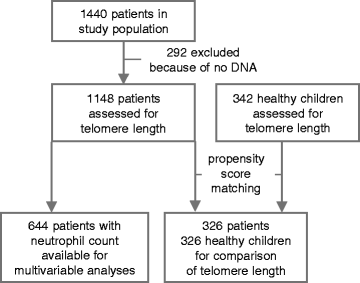
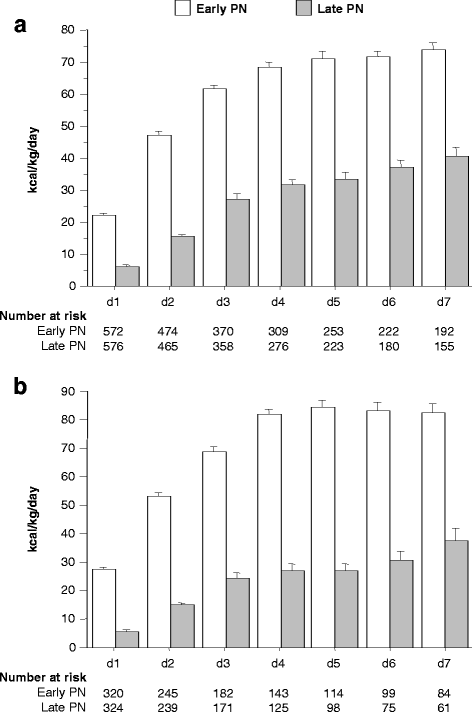
Methods: Telomere length was quantified in leukocyte DNA from 342 healthy children and from 1148 patients who had been enrolled in the multicenter, randomised controlled trial (RCT), PEPaNIC. These patients were randomly allocated to initiation of PN within 24 h (early PN) or to withholding PN for one week in PICU (late PN). The impact of early PN versus late PN on the change in telomere length from the first to last PICU-day was investigated with multivariable linear regression analyses.
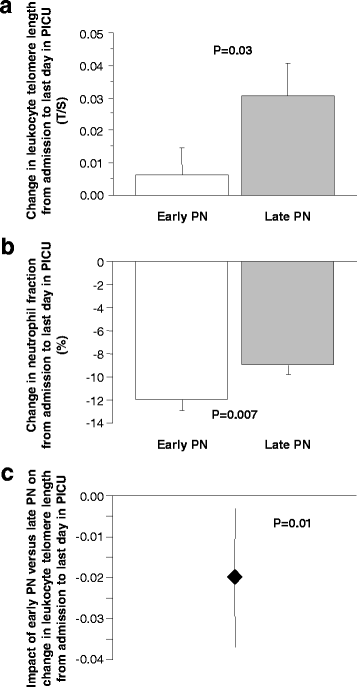
Results: Leukocyte telomeres were 6% shorter than normal upon PICU admission (median 1.625 (IQR 1.446-1.825) telomere/single-copy-gene ratio (T/S) units vs. 1.727 (1.547-1.915) T/S-units in healthy children (P < 0.0001)). Adjusted for potential baseline determinants and leukocyte composition, early PN was associated with telomere shortening during PICU stay as compared with late PN (estimate early versus late PN -0.021 T/S-units, 95% CI -0.038; 0.004, P = 0.01). Other independent determinants of telomere length identified in this model were age, gender, baseline telomere length and fraction of neutrophils in the sample from which the DNA was extracted. Telomere shortening with early PN was independent of post-randomisation factors affected by early PN, including longer length of PICU stay, larger amounts of insulin and higher risk of infection.
Conclusions: Shorter than normal leukocyte telomeres are present in critically ill children admitted to the PICU. Early initiation of PN further shortened telomeres, an effect that was independent of other determinants. Whether such telomere-shortening predisposes to long-term consequences of paediatric critical illness should be further investigated in a prospective follow-up study.
Trial registration: ClinicalTrials.gov, NCT01536275 . Registered on 16 February 2012.
Keywords: Children; Critical care; Critical illness; Intensive care; Nutrition; PICU; Paediatric; Telomere length; Telomeres.
Conflict of interest statement
Ethics approval and consent to participate
The institutional ethical review boards of the centres in Leuven (ML8052), Rotterdam (NL38772.000.12) and Edmonton (Pro00038098) approved the study, which was performed in accordance with the 1975 Declaration of Helsinki as revised in 1983. Written informed consent for participation in the trial, blood sampling and data analyses was obtained from the parents or legal guardians.
Consent for publication
Not applicable.
Competing interests
JL is a co-founder and consultant of Telomere Diagnostics. The company played no role in the current study. All other authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
On how to feed critically ill children in intensive care: A slowly shifting paradigm.Clin Nutr. 2025 Mar;46:169-180. doi: 10.1016/j.clnu.2025.02.003. Epub 2025 Feb 6. Clin Nutr. 2025. PMID: 39947042 Free PMC article. Review.
-
Outcomes of Delaying Parenteral Nutrition for 1 Week vs Initiation Within 24 Hours Among Undernourished Children in Pediatric Intensive Care: A Subanalysis of the PEPaNIC Randomized Clinical Trial.JAMA Netw Open. 2018 Sep 7;1(5):e182668. doi: 10.1001/jamanetworkopen.2018.2668. JAMA Netw Open. 2018. PMID: 30646158 Free PMC article. Clinical Trial.
-
Effect of late versus early initiation of parenteral nutrition on weight deterioration during PICU stay: Secondary analysis of the PEPaNIC randomised controlled trial.Clin Nutr. 2020 Jan;39(1):104-109. doi: 10.1016/j.clnu.2019.02.014. Epub 2019 Mar 4. Clin Nutr. 2020. PMID: 30879734 Clinical Trial.
-
Impact of withholding early parenteral nutrition completing enteral nutrition in pediatric critically ill patients (PEPaNIC trial): study protocol for a randomized controlled trial.Trials. 2015 May 1;16:202. doi: 10.1186/s13063-015-0728-8. Trials. 2015. PMID: 25927936 Free PMC article. Clinical Trial.
-
PN Administration in Critically Ill Children in Different Phases of the Stress Response.Nutrients. 2022 Apr 27;14(9):1819. doi: 10.3390/nu14091819. Nutrients. 2022. PMID: 35565787 Free PMC article. Review.
Cited by
-
Prenatal exposure to maternal psychological distress and telomere length in childhood.Dev Psychobiol. 2022 Jan;64(1):e22238. doi: 10.1002/dev.22238. Dev Psychobiol. 2022. PMID: 35050506 Free PMC article.
-
On how to feed critically ill children in intensive care: A slowly shifting paradigm.Clin Nutr. 2025 Mar;46:169-180. doi: 10.1016/j.clnu.2025.02.003. Epub 2025 Feb 6. Clin Nutr. 2025. PMID: 39947042 Free PMC article. Review.
-
Paediatric parenteral nutrition: current issues.Frontline Gastroenterol. 2019 Jul 9;11(2):148-154. doi: 10.1136/flgastro-2018-101127. eCollection 2020 Mar. Frontline Gastroenterol. 2019. PMID: 32133114 Free PMC article.
-
Early Supplemental Parenteral Nutrition in Critically Ill Children: An Update.J Clin Med. 2019 Jun 11;8(6):830. doi: 10.3390/jcm8060830. J Clin Med. 2019. PMID: 31212639 Free PMC article. Review.
-
Born to Age: When Adult Congenital Heart Disease Converges With Geroscience.JACC Adv. 2022 Mar 18;1(1):100012. doi: 10.1016/j.jacadv.2022.100012. eCollection 2022 Mar. JACC Adv. 2022. PMID: 38939088 Free PMC article. Review.
References
-
- Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373(9663):547–556. doi: 10.1016/S0140-6736(09)60044-1. - DOI - PubMed
-
- Mesotten D, Gielen M, Sterken C, Claessens K, Hermans G, Vlasselaers D, Lemiere J, Lagae L, Gewillig M, Eyskens B, et al. Neurocognitive development of children 4 years after critical illness and treatment with tight glucose control: a randomized controlled trial. JAMA. 2012;308(16):1641–1650. doi: 10.1001/jama.2012.12424. - DOI - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

