Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset
- PMID: 29463561
- PMCID: PMC5909763
- DOI: 10.1182/blood-2017-10-812891
Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset
Abstract
Conditioning-induced damage of the intestinal tract plays a critical role during the onset of acute graft-versus-host disease (GVHD). Therapeutic interference with these early events of GVHD is difficult, and currently used immunosuppressive drugs mainly target donor T cells. However, not donor T cells but neutrophils reach the sites of tissue injury first, and therefore could be a potential target for GVHD prevention. A detailed analysis of neutrophil fate during acute GVHD and the effect on T cells is difficult because of the short lifespan of this cell type. By using a novel photoconverter reporter system, we show that neutrophils that had been photoconverted in the ileum postconditioning later migrated to mesenteric lymph nodes (mLN). This neutrophil migration was dependent on the intestinal microflora. In the mLN, neutrophils colocalized with T cells and presented antigen on major histocompatibility complex (MHC)-II, thereby affecting T cell expansion. Pharmacological JAK1/JAK2 inhibition reduced neutrophil influx into the mLN and MHC-II expression, thereby interfering with an early event in acute GVHD pathogenesis. In agreement with this finding, neutrophil depletion reduced acute GVHD. We conclude that neutrophils are attracted to the ileum, where the intestinal barrier is disrupted, and then migrate to the mLN, where they participate in alloantigen presentation. JAK1/JAK2-inhibition can interfere with this process, which provides a potential therapeutic strategy to prevent early events of tissue damage-related innate immune cell activation and, ultimately, GVHD.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
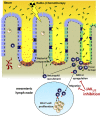
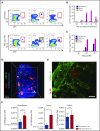
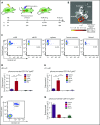
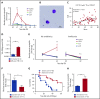


Comment in
-
Pathogenic neutrophils in acute GVHD.Blood. 2018 Apr 19;131(16):1774-1775. doi: 10.1182/blood-2018-03-836353. Blood. 2018. PMID: 29674354 No abstract available.
References
-
- Forman SJ, Negrin RS, Antin JH, Appelbaum FR, eds. Thomas’ Hematopoietic Cell Transplantation. 5th ed. Hoboken, NJ: Wiley-Blackwell; 2016.
-
- Gratwohl A, Pasquini MC, Aljurf M, et al. ; Worldwide Network for Blood and Marrow Transplantation (WBMT). One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;2(3):e91-e100. - PubMed
-
- Pasquini MC, Zhu X Current use and outcome of hematopoietic stem cell transplantation. Milwaukee, WI: 2015 CIBMTR Summary Slides; 2014.
-
- Arai S, Arora M, Wang T, et al. ; Graft-vs-Host Disease Working Committee of the CIBMTR. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(2):266-274. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

