β1-Integrin Impacts Rad51 Stability and DNA Double-Strand Break Repair by Homologous Recombination
- PMID: 29463647
- PMCID: PMC5902589
- DOI: 10.1128/MCB.00672-17
β1-Integrin Impacts Rad51 Stability and DNA Double-Strand Break Repair by Homologous Recombination
Abstract
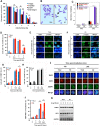
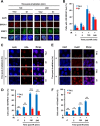
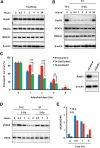
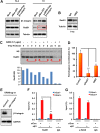
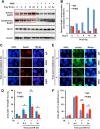
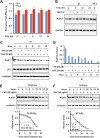
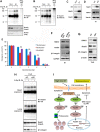
The molecular mechanisms underlying resistance to radiotherapy in breast cancer cells remain elusive. Previously, we reported that elevated β1-integrin is associated with enhanced breast cancer cell survival postirradiation, but how β1-integrin conferred radioresistance was unclear. Ionizing radiation (IR) induced cell killing correlates with the efficiency of DNA double-strand break (DSB) repair, and we found that nonmalignant breast epithelial (S1) cells with low β1-integrin expression have a higher frequency of S-phase-specific IR-induced chromosomal aberrations than the derivative malignant breast (T4-2) cells with high β1-integrin expression. In addition, there was an increased frequency of IR-induced homologous recombination (HR) repairosome focus formation in T4-2 cells compared with that of S1 cells. Cellular levels of Rad51 in T4-2 cells, a critical factor in HR-mediated DSB repair, were significantly higher. Blocking or depleting β1-integrin activity in T4-2 cells reduced Rad51 levels, while ectopic expression of β1-integrin in S1 cells correspondingly increased Rad51 levels, suggesting that Rad51 is regulated by β1-integrin. The low level of Rad51 protein in S1 cells was found to be due to rapid degradation by the ubiquitin proteasome pathway (UPP). Furthermore, the E3 ubiquitin ligase RING1 was highly upregulated in S1 cells compared to T4-2 cells. Ectopic β1-integrin expression in S1 cells reduced RING1 levels and increased Rad51 accumulation. In contrast, β1-integrin depletion in T4-2 cells significantly increased RING1 protein levels and potentiated Rad51 ubiquitination. These data suggest for the first time that elevated levels of the extracellular matrix receptor β1-integrin can increase tumor cell radioresistance by decreasing Rad51 degradation through a RING1-mediated proteasomal pathway.
Keywords: HR; Rad51; breast cancer; homologous recombination; radioresistance; β1-integrin.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O'Neil J, Gutierrez A, Ivanova E, Perna I, Lin E, Mani V, Jiang S, McNamara K, Zaghlul S, Edkins S, Stevens C, Brennan C, Martin ES, Wiedemeyer R, Kabbarah O, Nogueira C, Histen G, Aster J, Mansour M, Duke V, Foroni L, Fielding AK, Goldstone AH, Rowe JM, Wang YA, Look AT, Stratton MR, Chin L, Futreal PA, DePinho RA. 2007. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature 447:966–971. doi: 10.1038/nature05886. - DOI - PMC - PubMed
-
- Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, Haslett C. 1999. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med 5:662–668. doi: 10.1038/9511. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
