Novel Segment- and Host-Specific Patterns of Enteroaggregative Escherichia coli Adherence to Human Intestinal Enteroids
- PMID: 29463660
- PMCID: PMC5821088
- DOI: 10.1128/mBio.02419-17
Novel Segment- and Host-Specific Patterns of Enteroaggregative Escherichia coli Adherence to Human Intestinal Enteroids
Abstract
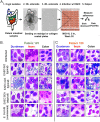
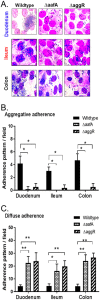
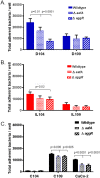
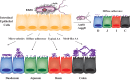
Enteroaggregative Escherichia coli (EAEC) is an important diarrheal pathogen and a cause of both acute and chronic diarrhea. It is a common cause of pediatric bacterial diarrhea in developing countries. Despite its discovery in 1987, the intestinal tropism of the pathogen remains unknown. Cell lines used to study EAEC adherence include the HEp-2, T-84, and Caco-2 lines, but they exhibit abnormal metabolism and large variations in gene expression. Animal models either do not faithfully manifest human clinical symptoms or are cumbersome and expensive. Using human intestinal enteroids derived from all four segments of the human intestine, we find that EAEC demonstrates aggregative adherence to duodenal and ileal enteroids, with donor-driven differences driving a sheet-like and layered pattern. This contrasts with the colon, where segment-specific tropisms yielded a mesh-like adherence pattern dominated by interconnecting filaments. Very little to no aggregative adherence to jejunal enteroids was observed, regardless of the strain or donor, in contrast to a strong duodenal association across all donors and strains. These unique patterns of intestinal segment- or donor-specific adherence, but not the overall numbers of associated bacteria, were dependent on the major subunit protein of aggregative adherence fimbriae II (AafA), implying that the morphology of adherent clusters and the overall intestinal cell association of EAEC occur by different mechanisms. Our results suggest that we must give serious consideration to inter- and intrapatient variations in what is arguably the first step in pathogenesis, that of adherence, when considering the clinical manifestation of these infections.IMPORTANCE EAEC is a leading cause of pediatric bacterial diarrhea and a common cause of diarrhea among travelers and immunocompromised individuals. Heterogeneity in EAEC strains and lack of a good model system are major roadblocks to the understanding of its pathogenesis. Utilizing human intestinal enteroids to study the adherence of EAEC, we demonstrate that unique patterns of adherence are largely driven by unidentified factors present in different intestinal segments and from different donors. These patterns are also dependent on aggregative adherence fimbriae II encoded by EAEC. These results imply that we must also consider the contribution of the host to understand the pathogenesis of EAEC-induced inflammation and diarrhea.
Keywords: adherence; enteroaggregative E. coli; enteroid; fimbriae; intestine; tropism.
Copyright © 2018 Rajan et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

