Structural basis and energy landscape for the Ca2+ gating and calmodulation of the Kv7.2 K+ channel
- PMID: 29463698
- PMCID: PMC5873240
- DOI: 10.1073/pnas.1800235115
Structural basis and energy landscape for the Ca2+ gating and calmodulation of the Kv7.2 K+ channel
Erratum in
-
Correction for Bernardo-Seisdedos et al., Structural basis and energy landscape for the Ca2+ gating and calmodulation of the Kv7.2 K+ channel.Proc Natl Acad Sci U S A. 2018 Apr 10;115(15):E3599. doi: 10.1073/pnas.1804137115. Epub 2018 Mar 26. Proc Natl Acad Sci U S A. 2018. PMID: 29581264 Free PMC article. No abstract available.
Abstract
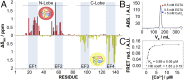
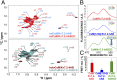
The Kv7.2 (KCNQ2) channel is the principal molecular component of the slow voltage-gated, noninactivating K+ M-current, a key controller of neuronal excitability. To investigate the calmodulin (CaM)-mediated Ca2+ gating of the channel, we used NMR spectroscopy to structurally and dynamically describe the association of helices hA and hB of Kv7.2 with CaM, as a function of Ca2+ concentration. The structures of the CaM/Kv7.2-hAB complex at two different calcification states are reported here. In the presence of a basal cytosolic Ca2+ concentration (10-100 nM), only the N-lobe of CaM is Ca2+-loaded and the complex (representative of the open channel) exhibits collective dynamics on the millisecond time scale toward a low-populated excited state (1.5%) that corresponds to the inactive state of the channel. In response to a chemical or electrical signal, intracellular Ca2+ levels rise up to 1-10 μM, triggering Ca2+ association with the C-lobe. The associated conformational rearrangement is the key biological signal that shifts populations to the closed/inactive channel. This reorientation affects the C-lobe of CaM and both helices in Kv7.2, allosterically transducing the information from the Ca2+-binding site to the transmembrane region of the channel.
Keywords: Kv7 potassium channel; M-current; calcium regulation; calmodulin; ion channel.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jespersen T, Grunnet M, Olesen S-P. The KCNQ1 potassium channel: From gene to physiological function. Physiology (Bethesda) 2005;20:408–416. - PubMed
-
- Sanguinetti MC, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. - PubMed
-
- Berkefeld H, et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. - PubMed
-
- Chin D, Means A-R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

