Bone degradation machinery of osteoclasts: An HIV-1 target that contributes to bone loss
- PMID: 29463701
- PMCID: PMC5856515
- DOI: 10.1073/pnas.1713370115
Bone degradation machinery of osteoclasts: An HIV-1 target that contributes to bone loss
Abstract
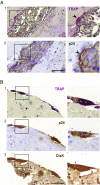
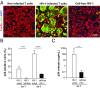
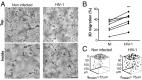
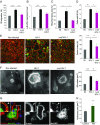
Bone deficits are frequent in HIV-1-infected patients. We report here that osteoclasts, the cells specialized in bone resorption, are infected by HIV-1 in vivo in humanized mice and ex vivo in human joint biopsies. In vitro, infection of human osteoclasts occurs at different stages of osteoclastogenesis via cell-free viruses and, more efficiently, by transfer from infected T cells. HIV-1 infection markedly enhances adhesion and osteolytic activity of human osteoclasts by modifying the structure and function of the sealing zone, the osteoclast-specific bone degradation machinery. Indeed, the sealing zone is broader due to F-actin enrichment of its basal units (i.e., the podosomes). The viral protein Nef is involved in all HIV-1-induced effects partly through the activation of Src, a regulator of podosomes and of their assembly as a sealing zone. Supporting these results, Nef-transgenic mice exhibit an increased osteoclast density and bone defects, and osteoclasts derived from these animals display high osteolytic activity. Altogether, our study evidences osteoclasts as host cells for HIV-1 and their pathological contribution to bone disorders induced by this virus, in part via Nef.
Keywords: HIV-1 infection; Nef; bone loss; osteoclast; podosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



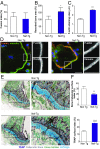
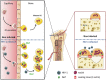
Comment in
-
Deciphering how HIV-1 weakens and cracks the bone.Proc Natl Acad Sci U S A. 2018 Mar 13;115(11):2551-2553. doi: 10.1073/pnas.1801555115. Epub 2018 Mar 1. Proc Natl Acad Sci U S A. 2018. PMID: 29496959 Free PMC article. No abstract available.
References
-
- Cotter AG, Mallon PW. The effects of untreated and treated HIV infection on bone disease. Curr Opin HIV AIDS. 2014;9:17–26. - PubMed
-
- Descours B, et al. CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature. 2017;543:564–567, and erratum (2017) 546:686. - PubMed
-
- Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 2003;17:1917–1923. - PubMed
-
- Gibellini D, et al. RANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV-1-positive men. J Med Virol. 2007;79:1446–1454. - PubMed
-
- Grijsen ML, et al. High prevalence of reduced bone mineral density in primary HIV-1-infected men. AIDS. 2010;24:2233–2238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

