Carbon monoxide protects the kidney through the central circadian clock and CD39
- PMID: 29463714
- PMCID: PMC5877926
- DOI: 10.1073/pnas.1716747115
Carbon monoxide protects the kidney through the central circadian clock and CD39
Abstract
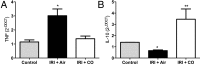
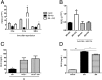
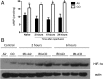
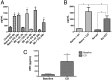
Ischemia reperfusion injury (IRI) is the predominant tissue insult associated with organ transplantation. Treatment with carbon monoxide (CO) modulates the innate immune response associated with IRI and accelerates tissue recovery. The mechanism has been primarily descriptive and ascribed to the ability of CO to influence inflammation, cell death, and repair. In a model of bilateral kidney IRI in mice, we elucidate an intricate relationship between CO and purinergic signaling involving increased CD39 ectonucleotidase expression, decreased expression of Adora1, with concomitant increased expression of Adora2a/2b. This response is linked to a >20-fold increase in expression of the circadian rhythm protein Period 2 (Per2) and a fivefold increase in serum erythropoietin (EPO), both of which contribute to abrogation of kidney IRI. CO is ineffective against IRI in Cd39-/- and Per2-/- mice or in the presence of a neutralizing antibody to EPO. Collectively, these data elucidate a cellular signaling mechanism whereby CO modulates purinergic responses and circadian rhythm to protect against injury. Moreover, these effects involve CD39- and adenosinergic-dependent stabilization of Per2. As CO also increases serum EPO levels in human volunteers, these findings continue to support therapeutic use of CO to treat IRI in association with organ transplantation, stroke, and myocardial infarction.
Keywords: DAMPS; adenosine; circadian rhythm; heme oxygenase; innate immunity.
Conflict of interest statement
Conflict of interest statement: L.E.O. is a scientific consultant for Hillhurst Biopharmaceuticals and has stock options. E.G. is a founder of Hillhurst Biopharmaceuticals and owns stock.
Figures





References
-
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. - PubMed
-
- Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 2009;87:859–864. - PubMed
-
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

