Diagnostic utility of telomere length testing in a hospital-based setting
- PMID: 29463756
- PMCID: PMC5877993
- DOI: 10.1073/pnas.1720427115
Diagnostic utility of telomere length testing in a hospital-based setting
Erratum in
-
Correction for Alder et al., Diagnostic utility of telomere length testing in a hospital-based setting.Proc Natl Acad Sci U S A. 2018 May 1;115(18):E4312. doi: 10.1073/pnas.1805407115. Epub 2018 Apr 23. Proc Natl Acad Sci U S A. 2018. PMID: 29686093 Free PMC article. No abstract available.
Abstract
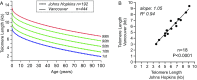
Telomere length (TL) predicts the onset of cellular senescence in vitro but the diagnostic utility of TL measurement in clinical settings is not fully known. We tested the value of TL measurement by flow cytometry and FISH (flowFISH) in patients with mutations in telomerase and telomere maintenance genes. TL had a discrete and reproducible normal range with definable upper and lower boundaries. While TL above the 50th age-adjusted percentile had a 100% negative predictive value for clinically relevant mutations, the lower threshold in mutation carriers was age-dependent, and adult mutation carriers often overlapped with the lowest decile of controls. The extent of telomere shortening correlated with the age at diagnosis as well as the short telomere syndrome phenotype. Extremely short TL caused bone marrow failure and immunodeficiency in children and young adults, while milder defects manifested as pulmonary fibrosis-emphysema in adults. We prospectively examined whether TL altered treatment decisions for newly diagnosed idiopathic bone marrow failure patients and found abnormally short TL enriched for patients with mutations in some inherited bone marrow failure genes, such as RUNX1, in addition to telomerase and telomere maintenance genes. The result was actionable, altering the choice of treatment regimen and/or hematopoietic stem cell donor in one-fourth of the cases (9 of 38, 24%). We conclude that TL measurement by flowFISH, when used for targeted clinical indications and in limited settings, can influence treatment decisions in ways that improve outcome.
Keywords: aplastic anemia; interstitial lung disease; liver disease; precision medicine; primary immunodeficiency.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. - PubMed
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. - PubMed
-
- d’Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. - PubMed
-
- Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

