Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage
- PMID: 29463838
- PMCID: PMC5820248
- DOI: 10.1038/s41598-018-21589-2
Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage
Abstract
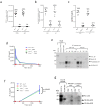
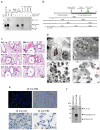
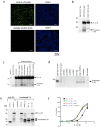
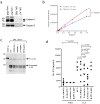
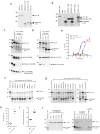
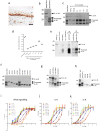
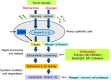
Interleukin (IL)-33 is an IL-1 family alarmin released from damaged epithelial and endothelial barriers to elicit immune responses and allergic inflammation via its receptor ST2. Serine proteases released from neutrophils, mast cells and cytotoxic lymphocytes have been proposed to process the N-terminus of IL-33 to enhance its activity. Here we report that processing of full length IL-33 can occur in mice deficient in these immune cell protease activities. We sought alternative mechanisms for the proteolytic activation of IL-33 and discovered that exogenous allergen proteases and endogenous calpains, from damaged airway epithelial cells, can process full length IL-33 and increase its alarmin activity up to ~60-fold. Processed forms of IL-33 of apparent molecular weights ~18, 20, 22 and 23 kDa, were detected in human lungs consistent with some, but not all, proposed processing sites. Furthermore, allergen proteases degraded processed forms of IL-33 after cysteine residue oxidation. We suggest that IL-33 can sense the proteolytic and oxidative microenvironment during tissue injury that facilitate its rapid activation and inactivation to regulate the duration of its alarmin function.
Conflict of interest statement
Authors that are employees of the AstraZeneca Group have stock/stock options in AstraZeneca.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

