Enhanced Biological Response of AVS-Functionalized Ti-6Al-4V Alloy through Covalent Immobilization of Collagen
- PMID: 29463865
- PMCID: PMC5820288
- DOI: 10.1038/s41598-018-21685-3
Enhanced Biological Response of AVS-Functionalized Ti-6Al-4V Alloy through Covalent Immobilization of Collagen
Abstract
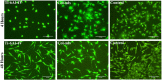
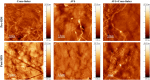
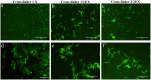
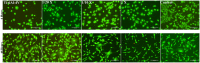
This study presents the development of an efficient procedure for covalently immobilizing collagen molecules on AVS-functionalized Ti-6Al-4V samples, and the assessment of the survival and proliferation of cells cultured on these substrates. Activated Vapor Silanization (AVS) is a versatile functionalization technique that allows obtaining a high density of active amine groups on the surface. A procedure is presented to covalently bind collagen to the functional layer using EDC/NHS as cross-linker. The covalently bound collagen proteins are characterized by fluorescence microscopy and atomic force microscopy and their stability is tested. The effect of the cross-linker concentration on the process is assessed. The concentration of the cross-linker is optimized and a reliable cleaning protocol is developed for the removal of the excess of carbodiimide from the samples. The results demonstrate that the covalent immobilization of collagen type I on Ti-6Al-4V substrates, using the optimized protocol, increases the number of viable cells present on the material. Consequently, AVS in combination with the carbodiimide chemistry appears as a robust method for the immobilization of proteins and, for the first time, it is shown that it can be used to enhance the biological response to the material.
Conflict of interest statement
The authors declare no competing interests.
Figures



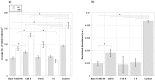

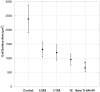
References
-
- Geetha M, Singh AK, Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants - A review. Progress in Materials Science. 2009;54:397–425. doi: 10.1016/j.pmatsci.2008.06.004. - DOI
-
- Brunette, D. M., Tengvall, P., Textor, M. & Thomsen, P. In Titanium in Medicine: MaterialScience, Surface Science, Engineering, Biological Responses and Medical Applications (Springer Science & Business Media, 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

