All-in-One Theranostic Nanoplatform Based on Hollow MoSx for Photothermally-maneuvered Oxygen Self-enriched Photodynamic Therapy
- PMID: 29463993
- PMCID: PMC5817104
- DOI: 10.7150/thno.22325
All-in-One Theranostic Nanoplatform Based on Hollow MoSx for Photothermally-maneuvered Oxygen Self-enriched Photodynamic Therapy
Abstract
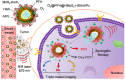
Photodynamic therapy (PDT) kills cancer cells by converting tumor-dissolved oxygen into reactive singlet oxygen (1O2) using a photosensitizer under laser irradiation. However, pre-existing hypoxia in tumors and oxygen consumption during PDT can result in an inadequate oxygen supply, which in turn hampers PDT efficacy. Herein, an O2 self-sufficient nanotheranostic platform based on hollow MoSx nanoparticles (HMoSx) with oxygen-saturated perfluorohexane (O2@PFH) and surface-modified human serum albumin (HSA)/chloride aluminium phthalocyanine (AlPc) (O2@PFH@HMoSx-HSA/AlPc), has been designed for the imaging and oxygen self-enriched photodynamic therapy (Oxy-PDT) of cancer.
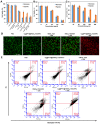
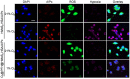
Methods: The in vitro anti-cancer activity and intracellular 1O2 generation performance of the nanoparticles were examined using 4T1 cells. We also evaluated the multimodal imaging capabilities and anti-tumor efficiency of the prepared nanoparticles in vivo using a 4T1 tumor-bearing nude mouse model.
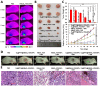
Results: This nanoplatform could achieve the distinct in vivo fluorescence (FL)/photoacoustic (PA)/X-ray computed tomography (CT) triple-model imaging-guided photothermally-maneuvered Oxy-PDT. Interestingly, the fluorescence and Oxy-PDT properties of O2@PFH@HMoSx-HSA/AlPc were considerably quenched; however, photothermal activation by 670 nm laser irradiation induced a significant increase in temperature, which empowered the Oxy-PDT effect of the nanoparticles. In this study, O2@PFH@HMoSx-HSA/AlPc demonstrated a great potential to image and treat tumors both in vitro and in vivo, showing complete tumor-inhibition over 16 days after treatment in the 4T1 tumor model.
Conclusion: O2@PFH@HMoSx-HSA/AlPc is promising to be used as novel multifunctional theranostic nanoagent for triple-modal imaging as well as single wavelength NIR laser triggered PTT/Oxy-PDT synergistic therapy.
Keywords: Hollow structure; Oxygen self-enriched photodynamic therapy.; Photothermally maneuvered; Theranostic nanoagent; Triple-modal imaging.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Foster TH, Gao L. Dosimetry in photodynamic therapy: oxygen and the critical importance of capillary density. Radiat Res. 1992;130:379–83. - PubMed
-
- Song X, Chen Q, Liu Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015;8:340–54.
-
- Luo GF, Chen WH, Lei Q, Qiu WX, Liu YX, Cheng YJ. et al. A Triple-Collaborative Strategy for High-performance tumor therapy by multifunctional mesoporous silica-coated gold nanorods. Adv Funct Mater. 2016;26:4339–50.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

