[Neratinib + Valproate] exposure permanently reduces ERBB1 and RAS expression in 4T1 mammary tumors and enhances M1 macrophage infiltration
- PMID: 29464055
- PMCID: PMC5814195
- DOI: 10.18632/oncotarget.23681
[Neratinib + Valproate] exposure permanently reduces ERBB1 and RAS expression in 4T1 mammary tumors and enhances M1 macrophage infiltration
Abstract
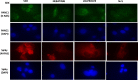
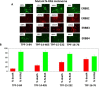
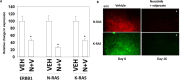
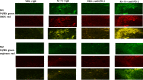
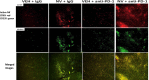
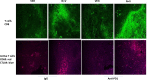
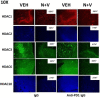
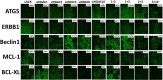
The irreversible ERBB1/2/4 inhibitor neratinib has been shown in vitro to rapidly reduce the expression of ERBB1/2/4 and RAS proteins via autophagic/lysosomal degradation. We have recently demonstrated that neratinib and valproate interact to suppress the growth of 4T1 mammary tumors but had not defined whether the [neratinib + valproate] drug combination, in a mouse, had altered the biology of the 4T1 cells. Exposure of 4T1 mammary tumors to [neratinib + valproate] for three days resulted, two weeks later, in tumors that expressed less ERBB1, K-RAS, N-RAS, indoleamine-pyrrole 2,3-dioxygenase (IDO-1), ornithine decarboxylase (ODC) and had increased Class I MHCA expression. Tumors previously exposed to [neratinib + valproate] grew more slowly than those exposed to vehicle control and contained more CD8+ cells and activated NK cells. M1 but not M2 macrophage infiltration was significantly enhanced by the drug combination. In vitro exposure of 4T1 tumor cells to [neratinib + valproate] variably reduced the expression of histone deacetylases 1-11. In vivo, prior exposure of tumors to [neratinib + valproate] permanently reduced the expression of HDACs 1-3, 6 and 10. Combined knock down of HDACs 1/2/3 or of 3/10 rapidly reduced the expression IDO-1, and ODC and increased the expression of MHCA. H&E staining of normal tissues at animal nadir revealed no obvious cyto-architectural differences between control and drug-treated animals. We conclude that [neratinib + valproate] evolves 4T1 tumors to grow more slowly and to be more sensitive to checkpoint immunotherapy antibodies.
Keywords: autophagy; neratinib; receptor tyrosine kinase; valproate.
Conflict of interest statement
CONFLICTS OF INTEREST None.
Figures




References
-
- Booth L, Roberts JL, Poklepovic A, Avogadri-Connors F, Cutler RE, Lalani AS, Dent P. HDAC inhibitors enhance neratinib activity and when combined enhance the actions of an anti-PD-1 immunomodulatory antibody in vivo. Oncotarget. 2017;8:90262–90277. https://doi.org/10.18632/oncotarget.21660. - DOI - PMC - PubMed
-
- Booth L, Roberts JL, Poklepovic A, Kirkwood J, Sander C, Avogadri-Connors F, Cutler RE, Lalani AS, Dent P. The levels of mutant K-RAS and mutant N-RAS are rapidly reduced by the irreversible ERBB1/2/4 inhibitor neratinib. Cancer Biol Ther. 2017 Dec 8 https://doi.org/10.1080/15384047.2017.1394556. [Epub ahead of print] - DOI - PMC - PubMed
-
- Zhang Y, Zhang J, Liu C, Du S, Feng L, Luan X, Zhang Y, Shi Y, Wang T, Wu Y, Cheng W, Meng S, Li M, et al. Neratinib induces ErbB2 ubiquitylation and endocytic degradation via HSP90 dissociation in breast cancer cells. Cancer Lett. 2016;382:176–185. - PubMed
-
- Booth L, Roberts JL, Sander C, Lee J, Kirkwood JM, Poklepovic A, Dent P. The HDAC inhibitor AR42 interacts with pazopanib to kill trametinib/dabrafenib-resistant melanoma cells in vitro and in vivo. Oncotarget. 2017;8:16367–16386. https://doi.org/10.18632/oncotarget.14829. - DOI - PMC - PubMed
-
- Booth L, Roberts JL, Poklepovic A, Gordon S, Dent P. PDE5 inhibitors enhance the lethality of pemetrexed through inhibition of multiple chaperone proteins and via the actions of cyclic GMP and nitric oxide. Oncotarget. 2017;8:1449–1468. https://doi.org/10.18632/oncotarget.13640. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

