Sequences Flanking the Gephyrin-Binding Site of GlyRβ Tune Receptor Stabilization at Synapses
- PMID: 29464196
- PMCID: PMC5818551
- DOI: 10.1523/ENEURO.0042-17.2018
Sequences Flanking the Gephyrin-Binding Site of GlyRβ Tune Receptor Stabilization at Synapses
Abstract
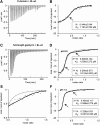
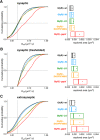
The efficacy of synaptic transmission is determined by the number of neurotransmitter receptors at synapses. Their recruitment depends upon the availability of postsynaptic scaffolding molecules that interact with specific binding sequences of the receptor. At inhibitory synapses, gephyrin is the major scaffold protein that mediates the accumulation of heteromeric glycine receptors (GlyRs) via the cytoplasmic loop in the β-subunit (β-loop). This binding involves high- and low-affinity interactions, but the molecular mechanism of this bimodal binding and its implication in GlyR stabilization at synapses remain unknown. We have approached this question using a combination of quantitative biochemical tools and high-density single molecule tracking in cultured rat spinal cord neurons. The high-affinity binding site could be identified and was shown to rely on the formation of a 310-helix C-terminal to the β-loop core gephyrin-binding motif. This site plays a structural role in shaping the core motif and represents the major contributor to the synaptic confinement of GlyRs by gephyrin. The N-terminal flanking sequence promotes lower affinity interactions by occupying newly identified binding sites on gephyrin. Despite its low affinity, this binding site plays a modulatory role in tuning the mobility of the receptor. Together, the GlyR β-loop sequences flanking the core-binding site differentially regulate the affinity of the receptor for gephyrin and its trapping at synapses. Our experimental approach thus bridges the gap between thermodynamic aspects of receptor-scaffold interactions and functional receptor stabilization at synapses in living cells.
Keywords: bimodal binding; binding site; gephyrin; glycine receptor; receptor clustering.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
