Antibiotics for treating gonorrhoea in pregnancy
- PMID: 29465747
- PMCID: PMC6491276
- DOI: 10.1002/14651858.CD011167.pub2
Antibiotics for treating gonorrhoea in pregnancy
Abstract
Background: Gonorrhoea is a sexually transmitted infection that is caused by Neisseria gonorrhoeae, and is a major public health challenge today. N gonorrhoeae can be transmitted from the mother's genital tract to the newborn during birth, and can cause gonococcal ophthalmia neonatorum as well as systemic neonatal infections. It can also cause endometritis and pelvic sepsis in the mother. This review updates and replaces an earlier Cochrane Review on antibiotics for treating this infectious condition.
Objectives: To assess the clinical effectiveness and harms of antibiotics for treating gonorrhoea in pregnant women.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2017), LILACS database (1982 to April 5, 2017), the WHO International Clinical Trials Registry Platform (ICTRP; April 5, 2017), ClinicalTrials.gov (April 5, 2017), the ISRCTN Registry (April 5, 2017), and Epistemonikos (April 5, 2017). We also searched reference lists of all retrieved articles.
Selection criteria: We included randomised controlled trials (RCTs) comparing the use of antibiotics for treating gonorrhoea in pregnancy. The antibiotics could have been used alone or in combination, were administered parenterally, orally, or both, and were compared with another antibiotic.We included RCTs regardless of their publication status (published, unpublished, published as an article, an abstract, or a letter), language, or country. We applied no limits on the length of follow-up.We excluded RCTs using a cluster- or cross-over design, or quasi-RCTs.
Data collection and analysis: Three review authors independently assessed trials for inclusion and risk of bias, extracted data, and checked them for accuracy.
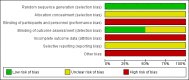
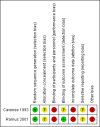
Main results: We included two RCTs, that randomised 514 pregnant women (347 women analysed) at a mean gestational age of 22 weeks. Both trials were conducted in the outpatient department of the same two hospitals in the USA between 1993 and 2001, and had a follow-up of 14 days. One of the trials was sponsored by a drug company. We considered both trials to be at a high risk of bias.One trial compared ceftriaxone (125 mg, intramuscular) with cefixime (400 mg, oral); the other trial had three arms, and assessed ceftriaxone (250 mg, intramuscular) versus either amoxicillin (3 g, oral) plus probenecid (1 g, oral) or spectinomycin (2 g, intramuscular). We did not include the spectinomycin data because this medication is no longer produced. We were unable to conduct meta-analysis because the trials compared different medications.We found inconclusive evidence that there were clear differences in the cure of gonococcal infections (genital, extragenital, or both) between intramuscular ceftriaxone versus oral amoxicillin plus oral probenecid (risk ratio (RR) 1.07, 95% confidence interval (CI) 0.98 to 1.16; one RCT; 168 women; very low-quality evidence) or intramuscular ceftriaxone versus oral cefixime (RR 0.99, 95% CI 0.91 to 1.08; one RCT; 95 women; very low-quality evidence).Neither of the trials reported on two of this review's primary maternal outcomes: incidence of obstetric complications (miscarriage, premature rupture of membranes, preterm delivery, or fetal death), or disseminated gonococcal infection, or on the incidence of neonatorum ophthalmia in the neonates.One trial reported one case of vomiting in the oral amoxacillin plus probenecid group. Trials reported pain at the injection sites, but did not quantify it. Hyperberbilurrubinemia was more frequent in neonates whose mothers were exposed to ceftriaxone. There were no clear differences between groups for neonatal malformation.
Authors' conclusions: This Cochrane Review found high levels of cure of gonococcal infections in pregnancy with the given antibiotic regimens. However, the evidence in this review is inconclusive as it does not support one particular regimen over another. This conclusion was based on very low-quality evidence (downgraded for poor trial design, imprecision) from two trials (involving 514 women), which we assessed to be at a high risk of bias for a number of domains. The harm profiles of the antibiotic regimes featured in this review remain unknown.High-quality RCTs are needed, with sufficient power to assess the clinical effectiveness and potential harms of antibiotics in pregnant women with gonorrhoea. These should be planned according to Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT),conducted following CONSORT recommendations, and based on Patient-Centered Outcomes Research Institute (PCORI) outcomes.
Conflict of interest statement
Gabriella Comunián‐Carrasco ‐ none known.
Guiomar E Peña‐Martí ‐ none known.
Arturo Martí‐Carvajal ‐ none known.
Figures
Update of
References
References to studies included in this review
Cavenee 1993 {published data only}
-
- Cavenee M, Ferris R, Rawlins S, Mayfield J, Wendel G. Treatment of gonorrhea in pregnancy. American Journal of Obstetrics and Gynecology 1991;164:300.
-
- Cavenee MR, Farris JR, Spalding TR, Barnes DL, Castaneda YS, Wendel GD. Treatment of gonorrhea in pregnancy. International Journal of Gynecology & Obstetrics 1993;43(1):93. - PubMed
-
- Cavenee MR, Farris JR, Spalding TR, Barnes DL, Castaneda YS, Wendel GD. Treatment of gonorrhea in pregnancy. Obstetrics & Gynecology 1993;81:33‐8. - PubMed
Ramus 2001 {published data only}
-
- Ramus R, Mayfield J, Wendel G. Evaluation of the current CDC recommended treatment guidelines for gonorrhea in pregnancy. American Journal of Obstetrics and Gynecology 1996;174:409.
-
- Ramus RM, Sheffield JS, Mayfield JA, Wendel GD. A randomized trial that compared oral cefixime and intramuscular ceftriaxone for the treatment of gonorrhea in pregnancy. American Journal of Obstetrics and Gynecology 2001;185(3):629‐32. - PubMed
References to studies excluded from this review
Shi 1997 {published data only}
-
- Shi DQ, Jiang JY. Effect observation of treating pregnancy gonorrhea with combination of western medicine and traditional Chinese medicine. Chinese Journal of Prevention and Control of STD and AIDS 1997;3(2):86.
Additional references
Altman 1995
Andes 2005
-
- Andes DR, Craig WA. Cephalosporins. In: Mandell GL, Bennett JE, Dolin R editor(s). Principles and Practice of Infectious Diseases. 6th Edition. Philadelphia: Churchill Livingstone, 2005:294–307.
Backmann 2017
-
- Backmann M. What's in a gold standard? In defence of randomised controlled trials. Medicine, Health Care, and Philosophy 2017;20(4):513‐23. [PUBMED: 28432483] - PubMed
Berger 2008
-
- Berger VW, Matthews JR, Grosch EN. On improving research methodology in clinical trials. Statistical Methods in Medical Research 2008;17(3):231‐42. [PUBMED: 17925317] - PubMed
Brunton 2018
-
- Brunton LL, Dandan RH, Knollmann BC, editors. Goodman and Gilman's Pharmacological Basis of Therapeutics. 13th Edition. New York: McGraw Hill, 2018. [ISBN 978‐1‐25‐958473‐2]
CDC 2015
-
- Centers for Disease Control and Prevention. Gonococcal Infections. Sexually Transmitted Diseases Treatment Guidelines. www.cdc.gov/std/tg2015/gonorrhea.htm (accessed 16 February 2018).
CDC 2016
-
- Centers for Disease Control and Prevention. Gonorrhea. Sexually Transmitted Diseases Surveillance. www.cdc.gov/std/stats16/Gonorrhea.htm (accessed 16 February 2018).
Chan 2013
Dechartres 2011
-
- Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single‐center trials show larger treatment effects than multicenter trials: evidence from a meta‐epidemiologic study. Annals of Internal Medicine 2011;155(1):39‐51. [PUBMED: 21727292] - PubMed
Deck 2009
-
- Deck DH, Winston LG. Tetracyclines, macrolides, clindamycin, chloramphenicol, streptogramins & oxazolidinones. In: Katzung BG, Masters SB, Trevor AJ editor(s). Basic & Clinical Pharmacology. 11th Edition. San Francisco: McGraw‐Hill, 2009:1019‐32. [IBSN: 978‐0‐07‐160405‐5]
Deck 2012
-
- Deck DH, Winston LG. Beta‐lactam & other cell wall‐ & membrane‐active antibiotics. In: Katzung BG, Masters SB, Trevor AJ editor(s). Basic & Clinical Pharmacology. 12th Edition. San Francisco: McGraw‐Hill, 2012:790‐8. [ISBN: 978‐0‐07‐176402‐5]
Frank 2014
-
- Frank L, Basch E, Selby JV. The PCORI perspective on patient‐centered outcomes research. JAMA 2014;312(15):1513‐4. [PUBMED: 25167382] - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Granowitz 2008
-
- Granowitz EV, Brown RB. Antibiotic adverse reactions and drug interactions. Critical Care Clinics 2008;24(2):421‐42. [PUBMED: 18361954] - PubMed
Guyatt 2008
Guyatt 2008a
Heather 2007
-
- Heather J, Lahra MM. The impact of infection during pregnancy on the mother and baby. In: Keeling JW editor(s). Fetal and Neonatal Pathology. 4th Edition. London: Springer Verlag, 2007:391‐2. [ISBN: 978‐1‐84628‐743‐5]
Higgins 2011
-
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2016
Ioannidis 2004
-
- Ioannidis JP, Evans SJ, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] - PubMed
Ison 2011
-
- Ison C. Biology of neisseria gonorrhoeae and the clinical picture of infections. Sexually Transmitted Infections and Sexually Transmitted Diseases. 1st Edition. London: Springer Verlag, 2001:79‐80. [ISBN: 978‐3‐642‐14663‐3]
Kreisel 2017
Lagace‐Wiens 2012
-
- Lagace‐Wiens P, Rubinstein E. Adverse reactions to betalactam antimicrobials. Expert Opinion on Drug Safety 2012;11(3):381‐99. [PUBMED: 22220627] - PubMed
Marrazzo 2015
-
- Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (Gonorrhea). In: Bennett JE, Dolin R, Blaser MJ editor(s). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th Edition. Philadelphia, PA: Saunders, an imprint of Elsevier Inc, 2015:2446‐62.e3. [ISBN 978‐0‐323‐40161‐6]
Mullick 2005
Nebeker 2004
-
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140(10):795‐801. [PUBMED: 15148066] - PubMed
Nguyen 2017
-
- Nguyen TL, Collins GS, Lamy A, Devereaux PJ, Daures JP, Landais P, et al. Simple randomization did not protect against bias in smaller trials. Journal of Clinical Epidemiology 2017;84:105‐13. [PUBMED: 28257927] - PubMed
Peterman 2016
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Satterwhite 2013
-
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sexually Transmitted Diseases 2013;40(3):187‐93. [PUBMED: 23403598] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Stefanelli 2011
-
- Stefanelli P. Emerging resistance in Neisseria meningitidis and Neisseria gonorrhoeae. Expert Review of Anti‐Infective Therapy 2011;9(2):237‐44. [PUBMED: 21342071] - PubMed
Torres 2010
-
- Torres MJ, Blanca M. The complex clinical picture of beta‐lactam hypersensitivity: penicillins, cephalosporins, monobactams, carbapenems, and clavams. Medical Clinics of North America 2010;94(4):805‐20. [PUBMED: 20609864] - PubMed
Turner 2012
-
- Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.MR000030.pub2; PUBMED: 23152285] - DOI - PMC - PubMed
Unverzagt 2013
-
- Unverzagt S, Prondzinsky R, Peinemann F. Single‐center trials tend to provide larger treatment effects than multicenter trials: a systematic review. Journal of Clinical Epidemiology 2013;66(11):1271‐80. [PUBMED: 23972520] - PubMed
WHO 2005
-
- World Health Organization (WHO). Sexually transmitted and other reproductive tract infections. hetv.org/resources/reproductive‐health/rtis_gep/rtis_gep.pdf (accessed June 2014).
WHO 2012
-
- World Health Organization (WHO). Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoea. whqlibdoc.who.int/publications/2012/9789241503501_eng.pdf (accessed June 2014).
WHO 2016
-
- World Health Organization (WHO). WHO guidelines for the treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea‐treatment‐gu... (accessed 6 April 2017). [ISBN 978 92 4 154969 1]
Woods 2005
-
- Woods CR. Gonococcal infections in neonates and young children. Seminars in Pediatric Infectious Diseases 2005;16(4):258‐70. - PubMed
Workowski 2010
-
- Workowski KA, Berman S, Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. Mortality and Morbidity Weekly Report 2010;59(RR12):1‐110. [PUBMED: 21160459] - PubMed
References to other published versions of this review
Brocklehurst 2002
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous