CK1ε and p120-catenin control Ror2 function in noncanonical Wnt signaling
- PMID: 29465811
- PMCID: PMC5928365
- DOI: 10.1002/1878-0261.12184
CK1ε and p120-catenin control Ror2 function in noncanonical Wnt signaling
Abstract
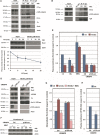
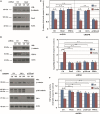
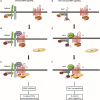
Canonical and noncanonical Wnt pathways share some common elements but differ in the responses they evoke. Similar to Wnt ligands acting through the canonical pathway, Wnts that activate the noncanonical signaling, such as Wnt5a, promote Disheveled (Dvl) phosphorylation and its binding to the Frizzled (Fz) Wnt receptor complex. The protein kinase CK1ε is required for Dvl/Fz association in both canonical and noncanonical signaling. Here we show that differently to its binding to canonical Wnt receptor complex, CK1ε does not require p120-catenin for the association with the Wnt5a co-receptor Ror2. Wnt5a promotes the formation of the Ror2-Fz complex and enables the activation of Ror2-bound CK1ε by Fz-associated protein phosphatase 2A. Moreover, CK1ε also regulates Ror2 protein levels; CK1ε association stabilizes Ror2, which undergoes lysosomal-dependent degradation in the absence of this kinase. Although p120-catenin is not required for CK1ε association with Ror2, it also participates in this signaling pathway as p120-catenin binds and maintains Ror2 at the plasma membrane; in p120-depleted cells, Ror2 is rapidly internalized through a clathrin-dependent mechanism. Accordingly, downregulation of p120-catenin or CK1ε affects late responses to Wnt5a that are also sensitive to Ror2, such as SIAH2 transcription, cell invasion, or cortical actin polarization. Our results explain how CK1ε is activated by noncanonical Wnt and identify p120-catenin and CK1ε as two critical factors controlling Ror2 function.
Keywords: CK1ε; Ror2; noncanonical Wnt; p120-catenin.
© 2018 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures




Similar articles
-
Role of the Ror family receptors in Wnt5a signaling.In Vitro Cell Dev Biol Anim. 2024 May;60(5):489-501. doi: 10.1007/s11626-024-00885-4. Epub 2024 Apr 8. In Vitro Cell Dev Biol Anim. 2024. PMID: 38587578 Review.
-
Negative regulation of Wnt signaling mediated by CK1-phosphorylated Dishevelled via Ror2.FASEB J. 2010 Jul;24(7):2417-26. doi: 10.1096/fj.09-150615. Epub 2010 Mar 9. FASEB J. 2010. PMID: 20215527
-
A p120-catenin-CK1epsilon complex regulates Wnt signaling.J Cell Sci. 2010 Aug 1;123(Pt 15):2621-31. doi: 10.1242/jcs.067512. J Cell Sci. 2010. PMID: 20940130
-
Canonical and non-canonical Wnt signaling are simultaneously activated by Wnts in colon cancer cells.Cell Signal. 2020 Aug;72:109636. doi: 10.1016/j.cellsig.2020.109636. Epub 2020 Apr 10. Cell Signal. 2020. PMID: 32283254
-
Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases.Dev Dyn. 2010 Jan;239(1):1-15. doi: 10.1002/dvdy.21991. Dev Dyn. 2010. PMID: 19530173 Review.
Cited by
-
The WNT/ROR Pathway in Cancer: From Signaling to Therapeutic Intervention.Cells. 2021 Jan 12;10(1):142. doi: 10.3390/cells10010142. Cells. 2021. PMID: 33445713 Free PMC article. Review.
-
Lef1 regulates caveolin expression and caveolin dependent endocytosis, a process necessary for Wnt5a/Ror2 signaling during Xenopus gastrulation.Sci Rep. 2019 Oct 30;9(1):15645. doi: 10.1038/s41598-019-52218-1. Sci Rep. 2019. PMID: 31666627 Free PMC article.
-
Repositioning of Etravirine as a Potential CK1ε Inhibitor by Virtual Screening.Pharmaceuticals (Basel). 2021 Dec 22;15(1):8. doi: 10.3390/ph15010008. Pharmaceuticals (Basel). 2021. PMID: 35056065 Free PMC article.
-
Role of the Ror family receptors in Wnt5a signaling.In Vitro Cell Dev Biol Anim. 2024 May;60(5):489-501. doi: 10.1007/s11626-024-00885-4. Epub 2024 Apr 8. In Vitro Cell Dev Biol Anim. 2024. PMID: 38587578 Review.
-
Intracellular Signals Activated by Canonical Wnt Ligands Independent of GSK3 Inhibition and β-Catenin Stabilization.Cells. 2019 Sep 25;8(10):1148. doi: 10.3390/cells8101148. Cells. 2019. PMID: 31557964 Free PMC article. Review.
References
-
- Alba‐Castellón L, Olivera‐Salguero R, Mestre‐Farrera A, Peña R, Herrera M, Bonilla F, Casal JI, Baulida J, Peña C and García de Herreros A (2016) Snail1‐dependent activation of cancer‐associated fibroblast controls epithelial tumor cell invasion and metastasis. Cancer Res 76, 6205–6217. - PubMed
-
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M and Niehrs C (2007) Wnt induces LRP6 signalosomes and promotes dishevelled‐dependent LRP6 phosphorylation. Science 316, 1619–1622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

