Development of Potent Inhibitors of Receptor Tyrosine Kinases by Ligand-Based Drug Design and Target-Biased Phenotypic Screening
- PMID: 29466002
- PMCID: PMC5851644
- DOI: 10.1021/acs.jmedchem.7b01605
Development of Potent Inhibitors of Receptor Tyrosine Kinases by Ligand-Based Drug Design and Target-Biased Phenotypic Screening
Abstract
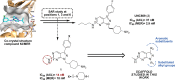
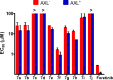
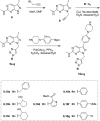
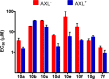
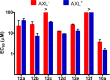
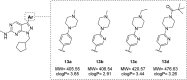
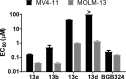
Pyrazolopyrimidines with potent antiproliferative properties were developed by an adaptive strategy that applies ligand-based design and phenotypic screening iteratively and is informed by biochemical assays. To drive development toward specific oncopathways, compounds were tested against cancer cells that overexpress, or not, AXL kinase. Identified phenotypic hits were found to inhibit oncotargets AXL, RET, and FLT3. Subsequent optimization generated antiproliferative lead compounds with unique selectivity profiles, including selective AXL inhibitors and a highly potent inhibitor of FLT3.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia.Invest New Drugs. 2017 Oct;35(5):556-565. doi: 10.1007/s10637-017-0470-z. Epub 2017 May 17. Invest New Drugs. 2017. PMID: 28516360 Free PMC article.
-
Inhibition of the receptor tyrosine kinase Axl impedes activation of the FLT3 internal tandem duplication in human acute myeloid leukemia: implications for Axl as a potential therapeutic target.Blood. 2013 Mar 14;121(11):2064-73. doi: 10.1182/blood-2012-07-444018. Epub 2013 Jan 15. Blood. 2013. PMID: 23321254 Free PMC article.
-
Design, synthesis, and biological evaluation of novel aminopyrimidinylisoindolines as AXL kinase inhibitors.Bioorg Med Chem Lett. 2018 Dec 15;28(23-24):3761-3765. doi: 10.1016/j.bmcl.2018.10.013. Epub 2018 Oct 11. Bioorg Med Chem Lett. 2018. PMID: 30340900
-
Role of the Receptor Tyrosine Kinase Axl and its Targeting in Cancer Cells.Curr Med Chem. 2016;23(15):1496-512. doi: 10.2174/0929867323666160405112954. Curr Med Chem. 2016. PMID: 27048336 Review.
-
AXL Inhibitors in Cancer: A Medicinal Chemistry Perspective.J Med Chem. 2016 Apr 28;59(8):3593-608. doi: 10.1021/acs.jmedchem.5b01273. Epub 2015 Nov 20. J Med Chem. 2016. PMID: 26555154 Review.
Cited by
-
Synthesis and Characterization of a Click-Assembled 18-Atom Macrocycle That Displays Selective AXL Kinase Inhibitory Activity.ACS Omega. 2019 Dec 3;4(25):21620-21626. doi: 10.1021/acsomega.9b03525. eCollection 2019 Dec 17. ACS Omega. 2019. PMID: 31867559 Free PMC article.
-
Recent developments in anticancer kinase inhibitors based on the pyrazolo[3,4-d]pyrimidine scaffold.RSC Med Chem. 2020 Sep 8;11(10):1112-1135. doi: 10.1039/d0md00227e. eCollection 2020 Oct 1. RSC Med Chem. 2020. PMID: 33479617 Free PMC article. Review.
-
Development of the phenylpyrazolo[3,4-d]pyrimidine-based, insulin-like growth factor receptor/Src/AXL-targeting small molecule kinase inhibitor.Theranostics. 2021 Jan 1;11(4):1918-1936. doi: 10.7150/thno.48865. eCollection 2021. Theranostics. 2021. PMID: 33408789 Free PMC article.
-
Discovery of pyrazolopyrimidines that selectively inhibit CSF-1R kinase by iterative design, synthesis and screening against glioblastoma cells.RSC Med Chem. 2023 Oct 11;14(12):2611-2624. doi: 10.1039/d3md00454f. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38099057 Free PMC article.
-
Pyrazolopyrimide library screening in glioma cells discovers highly potent antiproliferative leads that target the PI3K/mTOR pathway.Bioorg Med Chem. 2020 Jan 1;28(1):115215. doi: 10.1016/j.bmc.2019.115215. Epub 2019 Nov 25. Bioorg Med Chem. 2020. PMID: 31787462 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

