Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children
- PMID: 29466156
- PMCID: PMC5857389
- DOI: 10.1056/NEJMoa1714448
Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children
Abstract
Background: Fusions involving one of three tropomyosin receptor kinases (TRK) occur in diverse cancers in children and adults. We evaluated the efficacy and safety of larotrectinib, a highly selective TRK inhibitor, in adults and children who had tumors with these fusions.
Methods: We enrolled patients with consecutively and prospectively identified TRK fusion-positive cancers, detected by molecular profiling as routinely performed at each site, into one of three protocols: a phase 1 study involving adults, a phase 1-2 study involving children, or a phase 2 study involving adolescents and adults. The primary end point for the combined analysis was the overall response rate according to independent review. Secondary end points included duration of response, progression-free survival, and safety.
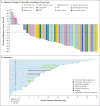
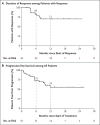
Results: A total of 55 patients, ranging in age from 4 months to 76 years, were enrolled and treated. Patients had 17 unique TRK fusion-positive tumor types. The overall response rate was 75% (95% confidence interval [CI], 61 to 85) according to independent review and 80% (95% CI, 67 to 90) according to investigator assessment. At 1 year, 71% of the responses were ongoing and 55% of the patients remained progression-free. The median duration of response and progression-free survival had not been reached. At a median follow-up of 9.4 months, 86% of the patients with a response (38 of 44 patients) were continuing treatment or had undergone surgery that was intended to be curative. Adverse events were predominantly of grade 1, and no adverse event of grade 3 or 4 that was considered by the investigators to be related to larotrectinib occurred in more than 5% of patients. No patient discontinued larotrectinib owing to drug-related adverse events.
Conclusions: Larotrectinib had marked and durable antitumor activity in patients with TRK fusion-positive cancer, regardless of the age of the patient or of the tumor type. (Funded by Loxo Oncology and others; ClinicalTrials.gov numbers, NCT02122913 , NCT02637687 , and NCT02576431 .).
Figures
Comment in
-
Developing Anticancer Drugs in Orphan Molecular Entities - A Paradigm under Construction.N Engl J Med. 2018 Feb 22;378(8):763-765. doi: 10.1056/NEJMe1716821. N Engl J Med. 2018. PMID: 29466154 No abstract available.
-
Larotrectinib in TRK fusion-positive cancers.Lancet Oncol. 2018 Apr;19(4):e187. doi: 10.1016/S1470-2045(18)30190-6. Epub 2018 Mar 1. Lancet Oncol. 2018. PMID: 29503244 No abstract available.
-
Targeted therapy: Larotrectinib effective against TRK-fusion-positive cancers.Nat Rev Clin Oncol. 2018 May;15(5):264. doi: 10.1038/nrclinonc.2018.40. Epub 2018 Mar 13. Nat Rev Clin Oncol. 2018. PMID: 29532798 No abstract available.
-
Breakthroughs and challenges in the management of tropomyosin receptor kinase fusion-positive tumors.Ann Transl Med. 2019 Jul;7(Suppl 3):S155. doi: 10.21037/atm.2019.06.41. Ann Transl Med. 2019. PMID: 31576362 Free PMC article. No abstract available.
References
-
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. - PubMed
-
- Russell JP, Powell DJ, Cunnane M, et al. The TRK-T1 fusion protein induces neoplastic transformation of thyroid epithelium. Oncogene. 2000;19:5729–35. - PubMed
-
- Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
