Human Neonatal Rotavirus Vaccine (RV3-BB) to Target Rotavirus from Birth
- PMID: 29466164
- PMCID: PMC5774175
- DOI: 10.1056/NEJMoa1706804
Human Neonatal Rotavirus Vaccine (RV3-BB) to Target Rotavirus from Birth
Abstract
Background: A strategy of administering a neonatal rotavirus vaccine at birth to target early prevention of rotavirus gastroenteritis may address some of the barriers to global implementation of a rotavirus vaccine.
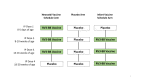
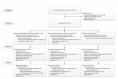
Methods: We conducted a randomized, double-blind, placebo-controlled trial in Indonesia to evaluate the efficacy of an oral human neonatal rotavirus vaccine (RV3-BB) in preventing rotavirus gastroenteritis. Healthy newborns received three doses of RV3-BB, administered according to a neonatal schedule (0 to 5 days, 8 weeks, and 14 weeks of age) or an infant schedule (8 weeks, 14 weeks, and 18 weeks of age), or placebo. The primary analysis was conducted in the per-protocol population, which included only participants who received all four doses of vaccine or placebo within the visit windows, with secondary analyses performed in the intention-to-treat population, which included all participants who underwent randomization.
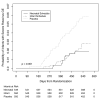
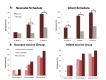
Results: Among the 1513 participants in the per-protocol population, severe rotavirus gastroenteritis occurred up to the age of 18 months in 5.6% of the participants in the placebo group (28 of 504 babies), in 1.4% in the neonatal-schedule vaccine group (7 of 498), and in 2.7% in the infant-schedule vaccine group (14 of 511). This resulted in a vaccine efficacy of 75% (95% confidence interval [CI], 44 to 91) in the neonatal-schedule group (P<0.001), 51% (95% CI, 7 to 76) in the infant-schedule group (P=0.03), and 63% (95% CI, 34 to 80) in the neonatal-schedule and infant-schedule groups combined (combined vaccine group) (P<0.001). Similar results were observed in the intention-to-treat analysis (1649 participants); the vaccine efficacy was 68% (95% CI, 35 to 86) in the neonatal-schedule group (P=0.001), 52% (95% CI, 11 to 76) in the infant-schedule group (P=0.02), and 60% (95% CI, 31 to 76) in the combined vaccine group (P<0.001). Vaccine response, as evidenced by serum immune response or shedding of RV3-BB in the stool, occurred in 78 of 83 participants (94%) in the neonatal-schedule group and in 83 of 84 participants (99%) in the infant-schedule group. The incidence of adverse events was similar across the groups. No episodes of intussusception occurred within the 21-day risk period after administration of any dose of vaccine or placebo, and one episode of intussusception occurred 114 days after the third dose of vaccine in the infant-schedule group.
Conclusions: RV3-BB was efficacious in preventing severe rotavirus gastroenteritis when administered according to a neonatal or an infant schedule in Indonesia. (Funded by the Bill and Melinda Gates Foundation and others; Australian New Zealand Clinical Trials Registry number, ACTRN12612001282875 .).
Conflict of interest statement
C.D.K, G.L.B. and R.F.B. and the MCRI hold a patent for the RV3-BB vaccine. C.D.K. (pre-2015) and J.E.B. (after 2015) have been/is the lead of the Australian Rotavirus Surveillance Program, which is supported by research grants from the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline, as well as the Australian Commonwealth Department of Health and Aging. From 2015 C.D.K. has been an employee of the Bill and Melinda Gates Foundation who provided funds to support this trial. J.E.B. is chair of a clinical events committee for a trial in Mali conducted by the University of Maryland and supported by Merck, she receives no payment although MCRI is compensated for her time. J.P.B. chairs influenza vaccine data safety monitoring boards for Sequiris Pty Ltd, the Australian distributor of RV5, he receives no payment although Monash Health is compensated for his time. N.S.B. is an employee of Bio Farma PT who contributed funds to UGM to support the conduct of this trial and plan to manufacture the RV3-BB vaccine. All other authors have no interest to declare.
Comment in
-
Rotavirus vaccine for neonates.Acta Paediatr. 2019 Apr;108(4):774. doi: 10.1111/apa.14697. Epub 2019 Jan 18. Acta Paediatr. 2019. PMID: 30548693 No abstract available.
References
-
- International Vaccine Access Center (IVAC). Vaccine Information Management System (VIMS) Global Rotavirus Vaccine Access Report. Johns Hopkins Bloomberg School of Public Health; 2017 [Accessed July 22, 2017, at http://www.view-hub.org/).
-
- Cherian T, Wang SS, Mantel C. Rotavirus vaccines in developing countries: The potential impact, implementation challenges, and remaining questions. Vaccine. 2012;30:A3-A6. - PubMed
-
- Das JK, Bhutta ZA. Global challenges in acute diarrhea. Curr Opin Gastroenterol. 2016;32(1):18-23. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical