Cross-reactive microbial peptides can modulate HIV-specific CD8+ T cell responses
- PMID: 29466365
- PMCID: PMC5821448
- DOI: 10.1371/journal.pone.0192098
Cross-reactive microbial peptides can modulate HIV-specific CD8+ T cell responses
Abstract
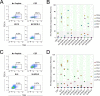
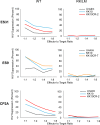
Heterologous immunity is an important aspect of the adaptive immune response. We hypothesized that this process could modulate the HIV-1-specific CD8+ T cell response, which has been shown to play an important role in HIV-1 immunity and control. We found that stimulation of peripheral blood mononuclear cells (PBMCs) from HIV-1-positive subjects with microbial peptides that were cross-reactive with immunodominant HIV-1 epitopes resulted in dramatic expansion of HIV-1-specific CD8+ T cells. Interestingly, the TCR repertoire of HIV-1-specific CD8+ T cells generated by ex vivo stimulation of PBMCs using HIV-1 peptide was different from that of cells stimulated with cross-reactive microbial peptides in some HIV-1-positive subjects. Despite these differences, CD8+ T cells stimulated with either HIV-1 or cross-reactive peptides effectively suppressed HIV-1 replication in autologous CD4+ T cells. These data suggest that exposure to cross-reactive microbial antigens can modulate HIV-1-specific immunity.
Conflict of interest statement
Figures




References
-
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283: 857–860. - PubMed
-
- Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3: 1061–1068. doi: 10.1038/ni845 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

