Roles of UVA radiation and DNA damage responses in melanoma pathogenesis
- PMID: 29466611
- PMCID: PMC6031472
- DOI: 10.1002/em.22176
Roles of UVA radiation and DNA damage responses in melanoma pathogenesis
Abstract
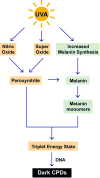
The growing incidence of melanoma is a serious public health issue that merits a thorough understanding of potential causative risk factors, which includes exposure to ultraviolet radiation (UVR). Though UVR has been classified as a complete carcinogen and has long been recognized for its ability to damage genomic DNA through both direct and indirect means, the precise mechanisms by which the UVA and UVB components of UVR contribute to the pathogenesis of melanoma have not been clearly defined. In this review, we therefore highlight recent studies that have addressed roles for UVA radiation in the generation of DNA damage and in modulating the subsequent cellular responses to DNA damage in melanocytes, which are the cell type that gives rise to melanoma. Recent research suggests that UVA not only contributes to the direct formation of DNA lesions but also impairs the removal of UV photoproducts from genomic DNA through oxidation and damage to DNA repair proteins. Moreover, the melanocyte microenvironment within the epidermis of the skin is also expected to impact melanomagenesis, and we therefore discuss several paracrine signaling pathways that have been shown to impact the DNA damage response in UV-irradiated melanocytes. Lastly, we examine how alterations to the immune microenvironment by UVA-associated DNA damage responses may contribute to melanoma development. Thus, there appear to be multiple avenues by which UVA may elevate the risk of melanoma. Protective strategies against excess exposure to UVA wavelengths of light therefore have the potential to decrease the incidence of melanoma. Environ. Mol. Mutagen. 59:438-460, 2018. © 2018 Wiley Periodicals, Inc.
Keywords: ATR kinase; DNA damage; DNA damage checkpoints; DNA damage signaling; DNA repair; Warburg effect; immune suppression; melanocytes; melanoma; nucleotide excision repair; skin cancer; ultraviolet A radiation; ultraviolet radiation.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

References
-
- Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, Ito S, Barsh G, Hearing VJ. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res. 2000;13(Suppl 8):156–162. - PubMed
-
- Abdel-Malek ZA, Ruwe A, Kavanagh-Starner R, Kadekaro AL, Swope V, Haskell-Luevano C, Koikov L, Knittel JJ. alpha-MSH tripeptide analogs activate the melanocortin 1 receptor and reduce UV-induced DNA damage in human melanocytes. Pigment Cell Melanoma Res. 2009;22:635–644. - PubMed
-
- Abraham RT. PI 3-kinase related kinases: 'big' players in stress-induced signaling pathways. DNA Repair (Amst) 2004;3:883–887. - PubMed
-
- Ando Y, Jensen PJ. Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J Invest Dermatol. 1993;100:633–639. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

