A Roadmap for Human Liver Differentiation from Pluripotent Stem Cells
- PMID: 29466743
- PMCID: PMC5854481
- DOI: 10.1016/j.celrep.2018.01.087
A Roadmap for Human Liver Differentiation from Pluripotent Stem Cells
Abstract
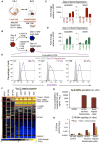
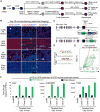
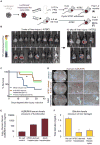
How are closely related lineages, including liver, pancreas, and intestines, diversified from a common endodermal origin? Here, we apply principles learned from developmental biology to rapidly reconstitute liver progenitors from human pluripotent stem cells (hPSCs). Mapping the formation of multiple endodermal lineages revealed how alternate endodermal fates (e.g., pancreas and intestines) are restricted during liver commitment. Human liver fate was encoded by combinations of inductive and repressive extracellular signals at different doses. However, these signaling combinations were temporally re-interpreted: cellular competence to respond to retinoid, WNT, TGF-β, and other signals sharply changed within 24 hr. Consequently, temporally dynamic manipulation of extracellular signals was imperative to suppress the production of unwanted cell fates across six consecutive developmental junctures. This efficiently generated 94.1% ± 7.35% TBX3+HNF4A+ human liver bud progenitors and 81.5% ± 3.2% FAH+ hepatocyte-like cells by days 6 and 18 of hPSC differentiation, respectively; the latter improved short-term survival in the Fah-/-Rag2-/-Il2rg-/- mouse model of liver failure.
Keywords: efficient differentiation; human liver development; pluripotent stem cells; progenitor; signaling.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



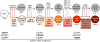
References
-
- Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. - PubMed
-
- Avior Y, Levy G, Zimerman M, Kitsberg D, Schwartz R, Sadeh R, Moussaieff A, Cohen M, Itskovitz-Eldor J, Nahmias Y. Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology. 2015;62:265–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

