Flexibility-Not just for yoga anymore!
- PMID: 29466861
- PMCID: PMC5890542
- DOI: 10.1177/2040206618756788
Flexibility-Not just for yoga anymore!
Abstract
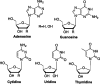
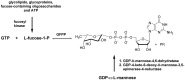
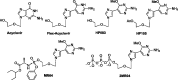
Over the past few years, nucleosides have maintained a prominent role as one of the cornerstones of antiviral and anticancer therapeutics, and many approaches to nucleoside drug design have been pursued. One such approach involves flexibility in the sugar moiety of nucleosides, for example, in the highly successful anti-HIV and HBV drug tenofovir. In contrast, introduction of flexibility to the nucleobase scaffold has only more recently gained significance with the invention of our fleximers. The history, development, and some biological relevance for this innovative class of nucleosides are detailed herein.
Keywords: Coronaviridae; dengue virus; filoviridae; flaviviridae; nucleoside analogues.
Figures


















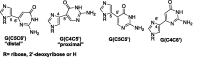
References
-
- Perigaud C Gosselin G andImbach JL. Nucleoside analogues as chemotherapeutic agents: a review. Nucleoside Nucleotides 1992; 11: 903–945.
-
- Jordheim LP, Durantel D, Zoulim F, et al. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov 2013; 12: 447–464. - PubMed
-
- De Clercq E. Strategies in the design of antiviral drugs. Nat Rev Drug Discov 2002; 1: 13–25. - PubMed
-
- De Clercq E. Antiviral drug discovery and development: where chemistry meets with biomedicine. Antiviral Res 2005; 67: 56–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

