Ginsenoside Re protects methamphetamine-induced dopaminergic neurotoxicity in mice via upregulation of dynorphin-mediated κ-opioid receptor and downregulation of substance P-mediated neurokinin 1 receptor
- PMID: 29467000
- PMCID: PMC5822489
- DOI: 10.1186/s12974-018-1087-7
Ginsenoside Re protects methamphetamine-induced dopaminergic neurotoxicity in mice via upregulation of dynorphin-mediated κ-opioid receptor and downregulation of substance P-mediated neurokinin 1 receptor
Abstract
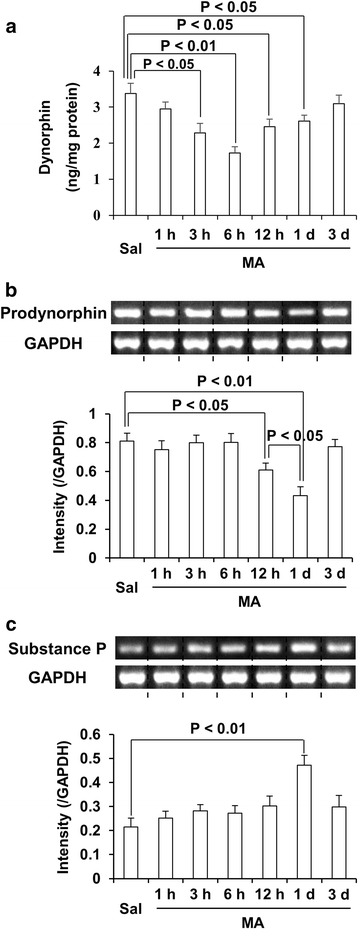
Background: We previously reported that ginsenoside Re (GRe) attenuated against methamphetamine (MA)-induced neurotoxicity via anti-inflammatory and antioxidant potentials. We also demonstrated that dynorphin possesses anti-inflammatory and antioxidant potentials against dopaminergic loss, and that balance between dynorphin and substance P is important for dopaminergic neuroprotection. Thus, we examined whether GRe positively affects interactive modulation between dynorphin and substance P against MA neurotoxicity in mice.
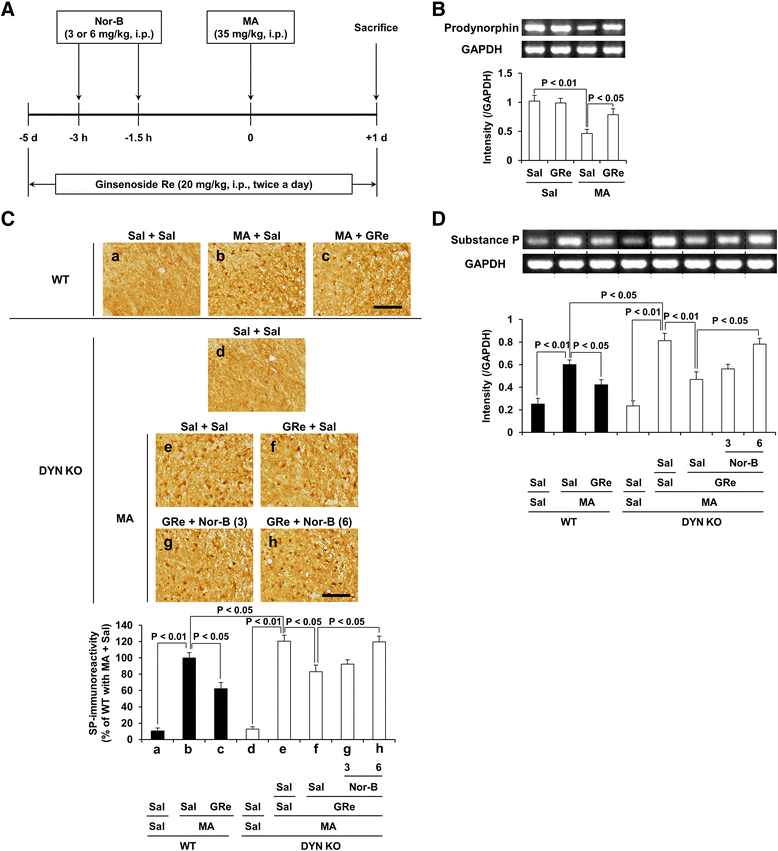
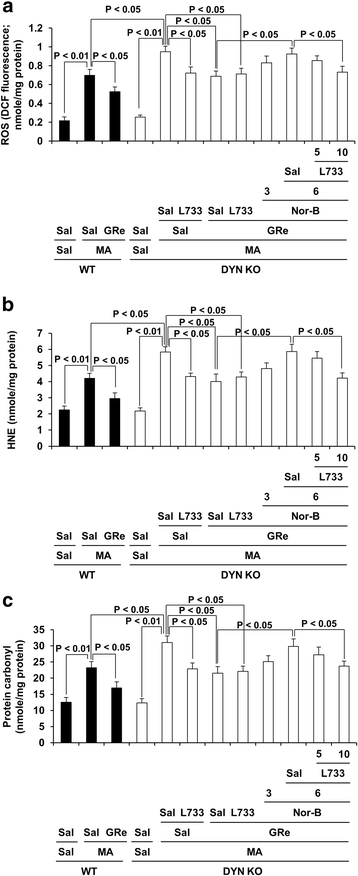
Methods: We examined changes in dynorphin peptide level, prodynorphin mRNA, and substance P mRNA, substance P-immunoreactivity, homeostasis in enzymatic antioxidant system, oxidative parameter, microglial activation, and pro-apoptotic parameter after a neurotoxic dose of MA to clarify the effects of GRe, prodynorphin knockout, pharmacological inhibition of κ-opioid receptor (i.e., nor-binaltorphimine), or neurokinin 1 (NK1) receptor (i.e., L-733,060) against MA insult in mice.
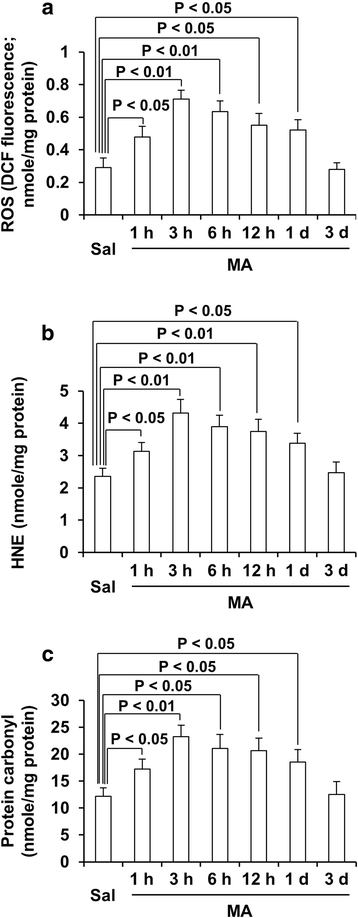
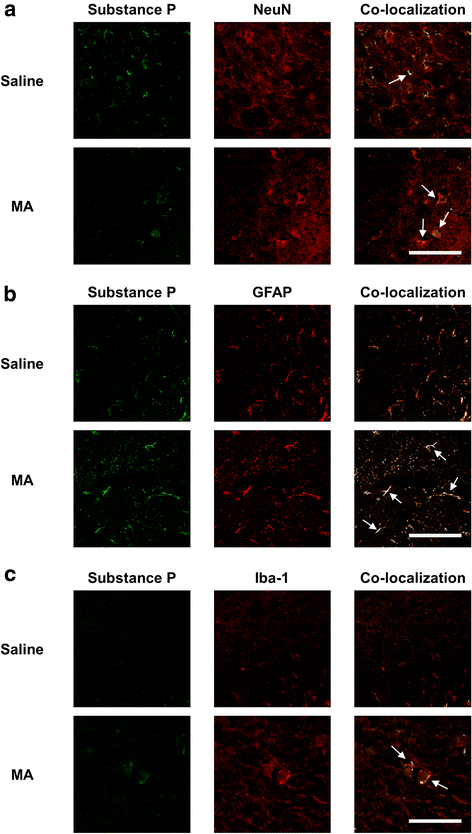
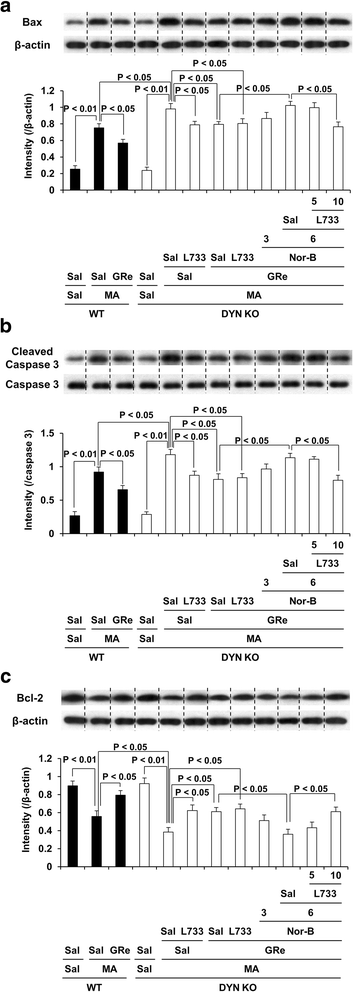
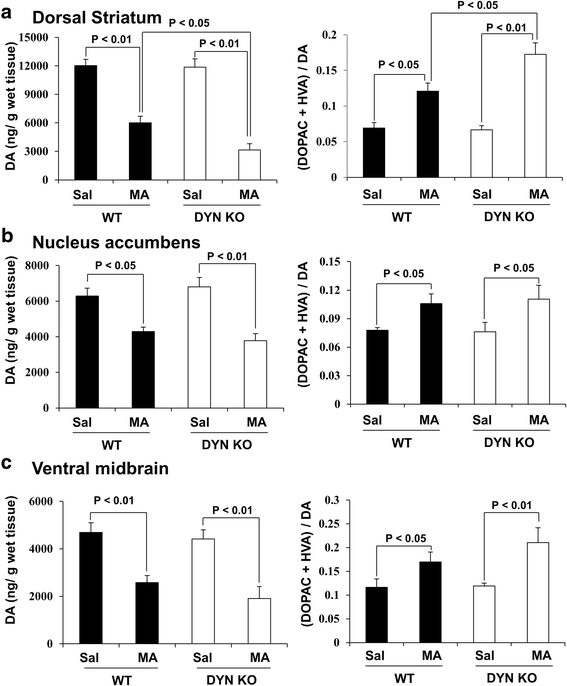
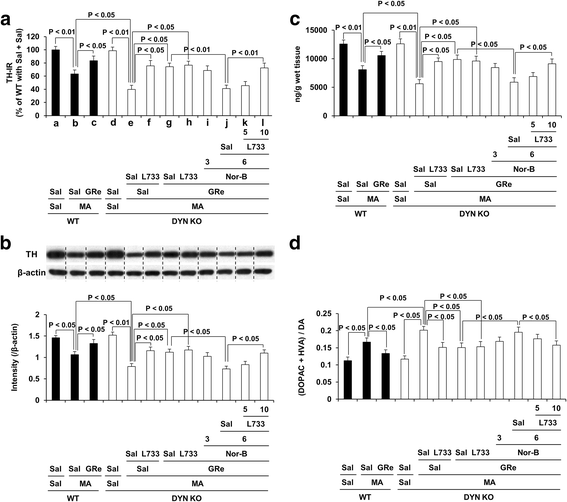
Results: GRe attenuated MA-induced decreases in dynorphin level, prodynorphin mRNA expression in the striatum of wild-type (WT) mice. Prodynorphin knockout potentiated MA-induced dopaminergic toxicity in mice. The imbalance of enzymatic antioxidant system, oxidative burdens, microgliosis, and pro-apoptotic changes led to the dopaminergic neurotoxicity. Neuroprotective effects of GRe were more pronounced in prodynorphin knockout than in WT mice. Nor-binaltorphimine, a κ-opioid receptor antagonist, counteracted against protective effects of GRe. In addition, we found that GRe significantly attenuated MA-induced increases in substance P-immunoreactivity and substance P mRNA expression in the substantia nigra. These increases were more evident in prodynorphin knockout than in WT mice. Although, we observed that substance P-immunoreactivity was co-localized in NeuN-immunreactive neurons, GFAP-immunoreactive astrocytes, and Iba-1-immunoreactive microglia. NK1 receptor antagonist L-733,060 or GRe selectively inhibited microgliosis induced by MA. Furthermore, L-733,060 did not show any additive effects against GRe-mediated protective activity (i.e., antioxidant, antimicroglial, and antiapoptotic effects), indicating that NK1 receptor is one of the molecular targets of GRe.
Conclusions: Our results suggest that GRe protects MA-induced dopaminergic neurotoxicity via upregulatgion of dynorphin-mediated κ-opioid receptor and downregulation of substance P-mediated NK1 R.
Keywords: Dynorphin; Methamphetamine; Microglia; Neurokinin 1 receptor; κ-opioid receptor.
Conflict of interest statement
Ethics approval
All animals were treated in accordance with the National Institutes of Health (NIH) Guide for the Humane Care and Use of Laboratory Animals (NIH Publication No. 85–23, 1985;
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

