Neuregulin-1 elicits a regulatory immune response following traumatic spinal cord injury
- PMID: 29467001
- PMCID: PMC5822667
- DOI: 10.1186/s12974-018-1093-9
Neuregulin-1 elicits a regulatory immune response following traumatic spinal cord injury
Abstract
Background: Spinal cord injury (SCI) triggers a robust neuroinflammatory response that governs secondary injury mechanisms with both degenerative and pro-regenerative effects. Identifying new immunomodulatory therapies to promote the supportive aspect of immune response is critically needed for the treatment of SCI. We previously demonstrated that SCI results in acute and permanent depletion of the neuronally derived Neuregulin-1 (Nrg-1) in the spinal cord. Increasing the dysregulated level of Nrg-1 through acute intrathecal Nrg-1 treatment enhanced endogenous cell replacement and promoted white matter preservation and functional recovery in rat SCI. Moreover, we identified a neuroprotective role for Nrg-1 in moderating the activity of resident astrocytes and microglia following injury. To date, the impact of Nrg-1 on immune response in SCI has not yet been investigated. In this study, we elucidated the effect of systemic Nrg-1 therapy on the recruitment and function of macrophages, T cells, and B cells, three major leukocyte populations involved in neuroinflammatory processes following SCI.
Methods: We utilized a clinically relevant model of moderately severe compressive SCI in female Sprague-Dawley rats. Nrg-1 (2 μg/day) or saline was delivered subcutaneously through osmotic mini-pumps starting 30 min after SCI. We conducted flow cytometry, quantitative real-time PCR, and immunohistochemistry at acute, subacute, and chronic stages of SCI to investigate the effects of Nrg-1 treatment on systemic and spinal cord immune response as well as cytokine, chemokine, and antibody production.
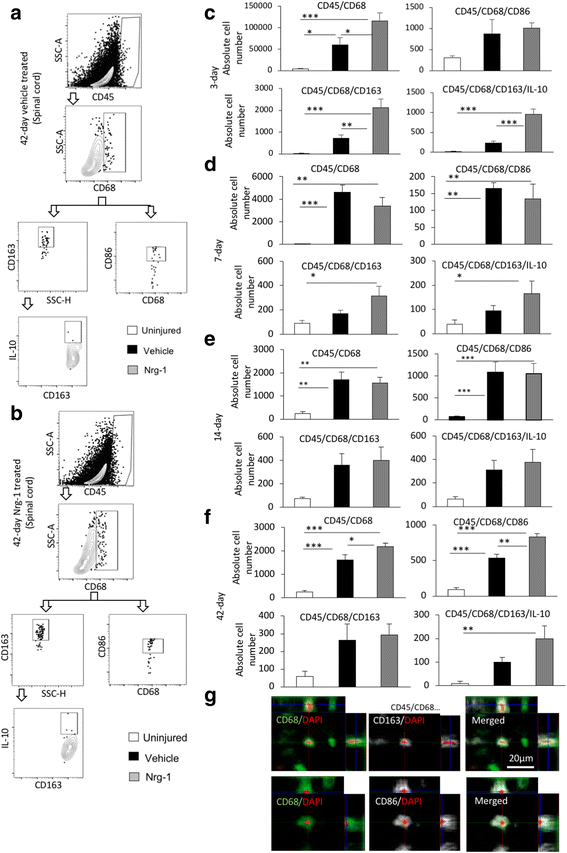
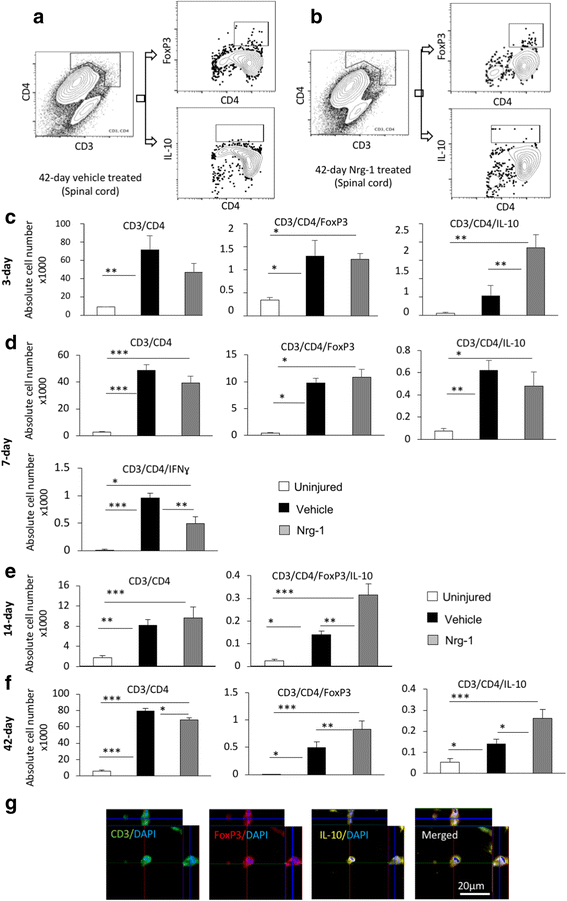
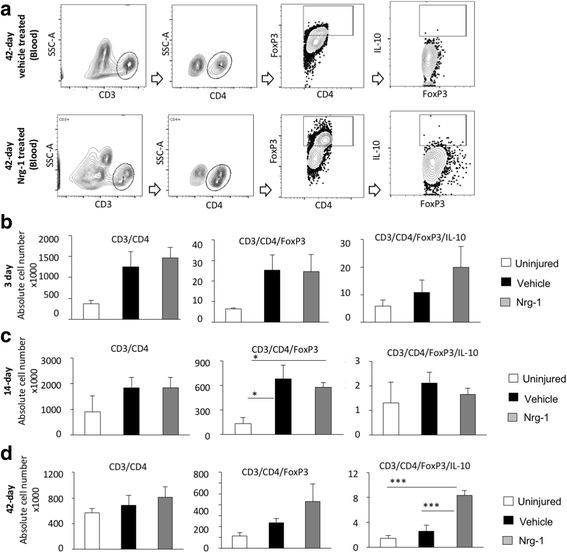
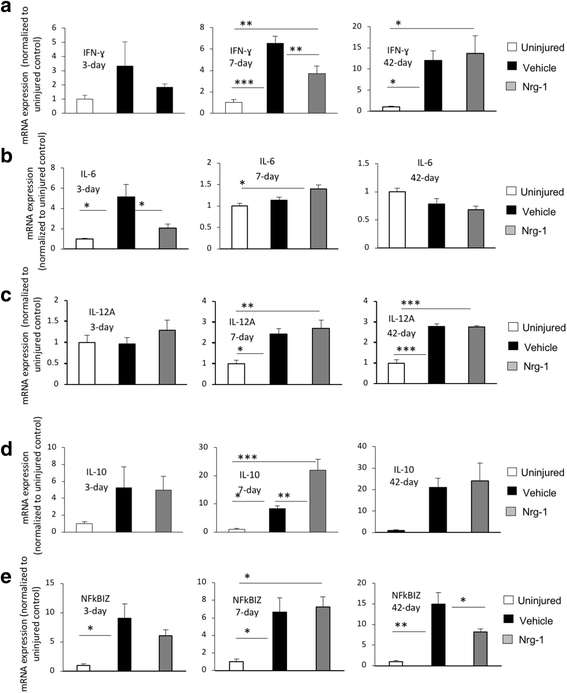
Results: We provide novel evidence that Nrg-1 promotes a pro-regenerative immune response after SCI. Bioavailability of Nrg-1 stimulated a regulatory phenotype in T and B cells and augmented the population of M2 macrophages in the spinal cord and blood during the acute and chronic stages of SCI. Importantly, Nrg-1 fostered a more balanced microenvironment in the injured spinal cord by attenuating antibody deposition and expression of pro-inflammatory cytokines and chemokines while upregulating pro-regenerative mediators.
Conclusion: We provide the first evidence of a significant regulatory role for Nrg-1 in neuroinflammation after SCI. Importantly, the present study establishes the promise of systemic Nrg-1 treatment as a candidate immunotherapy for traumatic SCI and other CNS neuroinflammatory conditions.
Keywords: Autoantibodies; B cells; Cytokines and chemokines; Macrophages; Neuregulin-1; Neuroinflammation; Rat; Spinal cord injury; T cells.
Conflict of interest statement
Ethics approval
All experimental protocols in this study involving animals were approved by the Animal Care Committee of University of Manitoba in accordance with the guidelines and policies established by the Canadian Council on Animal Care, protocol number: 15030 (AC11067).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

