Male synthetic sling versus artificial urinary sphincter trial for men with urodynamic stress incontinence after prostate surgery (MASTER): study protocol for a randomised controlled trial
- PMID: 29467024
- PMCID: PMC5822657
- DOI: 10.1186/s13063-018-2501-2
Male synthetic sling versus artificial urinary sphincter trial for men with urodynamic stress incontinence after prostate surgery (MASTER): study protocol for a randomised controlled trial
Abstract
Background: Stress urinary incontinence (SUI) is a frequent adverse effect for men undergoing prostate surgery. A large proportion (around 8% after radical prostatectomy and 2% after transurethral resection of prostate (TURP)) are left with severe disabling incontinence which adversely effects their quality of life and many are reliant on containment measures such as pads (27% and 6% respectively). Surgery is currently the only option for active management of the problem. The overwhelming majority of surgeries for persistent bothersome SUI involve artificial urinary sphincter (AUS) insertion. However, this is expensive, and necessitates manipulation of a pump to enable voiding. More recently, an alternative to AUS has been developed - a synthetic sling for men which elevates the urethra, thus treating SUI. This is thought, by some, to be less invasive, more acceptable and less expensive than AUS but clear evidence for this is lacking. The MASTER trial aims to determine whether the male synthetic sling is non-inferior to implantation of the AUS for men who have SUI after prostate surgery (for cancer or benign disease), judged primarily on clinical effectiveness but also considering relative harms and cost-effectiveness.
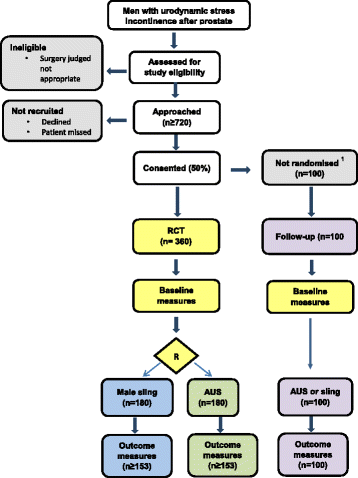
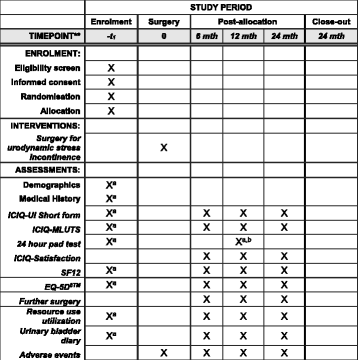
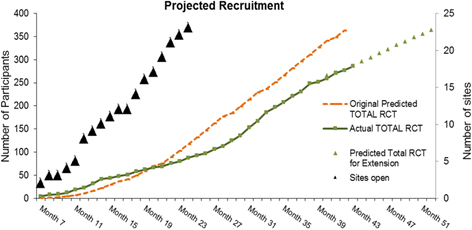
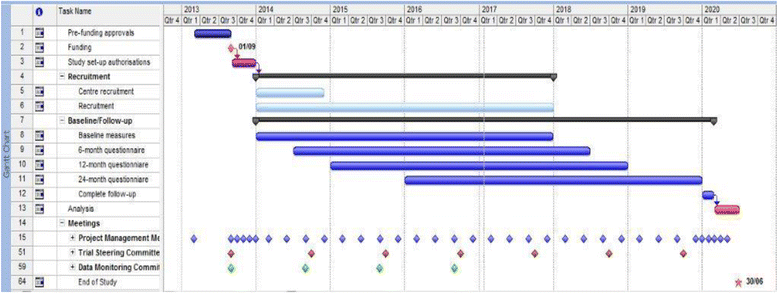
Methods/design: Men with urodynamic stress incontinence (USI) after prostate surgery, for whom surgery is judged appropriate, are the target population. We aim to recruit men from secondary care urological centres in the UK NHS who carry out surgery for post-prostatectomy incontinence. Outcomes will be assessed by participant-completed questionnaires and 3-day urinary bladder diaries at baseline, 6, 12 and 24 months. The 24-h urinary pad test will be used at baseline as an objective assessment of urine loss. Clinical data will be completed at the time of surgery to provide details of the operative procedures, complications and resource use in hospital. At 12 months, men will also have a clinical review to evaluate the results of surgery (including another 24-h pad test) and to identify problems or need for further treatment.
Discussion: A robust examination of the comparative effectiveness of the male synthetic sling will provide high-quality evidence to determine whether or not it should be adopted widely in the NHS.
Trial registration: International Standard Randomised Controlled Trial Registry: Number ISRCTN49212975 . Registered on 22 July 2013. First patient randomised on 29 January 2014.
Keywords: Artificial urinary sphincter; Male sling; Randomised controlled trial; Urinary incontinence.
Conflict of interest statement
Ethics approval and consent to participate
The NRES South West – Frenchay Research Ethics Committee has reviewed this study. The study will be conducted according to the principles of good practice provided by Research Governance Guidelines. We believe this study does not pose any specific risks to individual participants beyond those of any surgery, nor does it raise any extraordinary ethical issues.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Urinary incontinence in men after formal one-to-one pelvic-floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials. Lancet. 2011;378(9788):328–337. doi: 10.1016/S0140-6736(11)60751-4. - DOI - PubMed
-
- National Institute for Health and Clinical Excellence. NICE CG97 Lower urinary tract symptoms: full guidance [document on the Internet]. 2010. Available at: https://www.nice.org.uk/guidance/CG97. Accessed June 2012.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical