Stac proteins associate with the critical domain for excitation-contraction coupling in the II-III loop of CaV1.1
- PMID: 29467163
- PMCID: PMC5881444
- DOI: 10.1085/jgp.201711917
Stac proteins associate with the critical domain for excitation-contraction coupling in the II-III loop of CaV1.1
Abstract
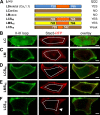
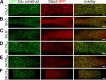
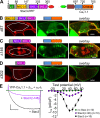
In skeletal muscle, residues 720-764/5 within the CaV1.1 II-III loop form a critical domain that plays an essential role in transmitting the excitation-contraction (EC) coupling Ca2+ release signal to the type 1 ryanodine receptor (RyR1) in the sarcoplasmic reticulum. However, the identities of proteins that interact with the loop and its critical domain and the mechanism by which the II-III loop regulates RyR1 gating remain unknown. Recent work has shown that EC coupling in skeletal muscle of fish and mice depends on the presence of Stac3, an adaptor protein that is highly expressed only in skeletal muscle. Here, by using colocalization as an indicator of molecular interactions, we show that Stac3, as well as Stac1 and Stac2 (predominantly neuronal Stac isoforms), interact with the II-III loop of CaV1.1. Further, we find that these Stac proteins promote the functional expression of CaV1.1 in tsA201 cells and support EC coupling in Stac3-null myotubes and that Stac3 is the most effective. Coexpression in tsA201 cells reveals that Stac3 interacts only with II-III loop constructs containing the majority of the CaV1.1 critical domain residues. By coexpressing Stac3 in dysgenic (CaV1.1-null) myotubes together with CaV1 constructs whose chimeric II-III loops had previously been tested for functionality, we reveal that the ability of Stac3 to interact with them parallels the ability of these constructs to mediate skeletal type EC coupling. Based on coexpression in tsA201 cells, the interaction of Stac3 with the II-III loop critical domain does not require the presence of the PKC C1 domain in Stac3, but it does require the first of the two SH3 domains. Collectively, our results indicate that activation of RyR1 Ca2+ release by CaV1.1 depends on Stac3 being bound to critical domain residues in the II-III loop.
© 2018 Polster et al.
Figures








References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

