Altered translation initiation of Gja1 limits gap junction formation during epithelial-mesenchymal transition
- PMID: 29467255
- PMCID: PMC5905293
- DOI: 10.1091/mbc.E17-06-0406
Altered translation initiation of Gja1 limits gap junction formation during epithelial-mesenchymal transition
Abstract
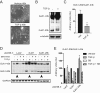
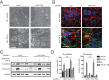
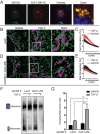
Epithelial-mesenchymal transition (EMT) is activated during development, wound healing, and pathologies including fibrosis and cancer metastasis. Hallmarks of EMT are remodeling of intercellular junctions and adhesion proteins, including gap junctions. The GJA1 mRNA transcript encoding the gap junction protein connexin43 (Cx43) has been demonstrated to undergo internal translation initiation, yielding truncated isoforms that modulate gap junctions. The PI3K/Akt/mTOR pathway is central to translation regulation and is activated during EMT, leading us to hypothesize that altered translation initiation would contribute to gap junction loss. Using TGF-β-induced EMT as a model, we find reductions in Cx43 gap junctions despite increased transcription and stabilization of Cx43 protein. Biochemical experiments reveal suppression of the internally translated Cx43 isoform, GJA1-20k in a Smad3 and ERK-dependent manner. Ectopic expression of GJA1-20k does not halt EMT, but is sufficient to rescue gap junction formation. GJA1-20k localizes to the Golgi apparatus, and using superresolution localization microscopy we find retention of GJA1-43k at the Golgi in mesenchymal cells lacking GJA1-20k. NativePAGE demonstrates that levels of GJA1-20k regulate GJA1-43k hexamer oligomerization, a limiting step in Cx43 trafficking. These findings reveal alterations in translation initiation as an unexplored mechanism by which the cell regulates Cx43 gap junction formation during EMT.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

