Oncogenic JAK2V617F causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms
- PMID: 29467301
- PMCID: PMC6034655
- DOI: 10.1126/scitranslmed.aam7729
Oncogenic JAK2V617F causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms
Abstract
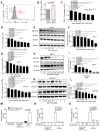
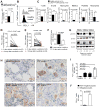
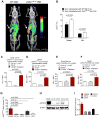
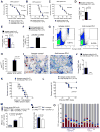
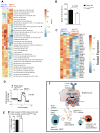
Recent evidence has revealed that oncogenic mutations may confer immune escape. A better understanding of how an oncogenic mutation affects immunosuppressive programmed death ligand 1 (PD-L1) expression may help in developing new therapeutic strategies. We show that oncogenic JAK2 (Janus kinase 2) activity caused STAT3 (signal transducer and activator of transcription 3) and STAT5 phosphorylation, which enhanced PD-L1 promoter activity and PD-L1 protein expression in JAK2V617F-mutant cells, whereas blockade of JAK2 reduced PD-L1 expression in myeloid JAK2V617F-mutant cells. PD-L1 expression was higher on primary cells isolated from patients with JAK2V617F-myeloproliferative neoplasms (MPNs) compared to healthy individuals and declined upon JAK2 inhibition. JAK2V617F mutational burden, pSTAT3, and PD-L1 expression were highest in primary MPN patient-derived monocytes, megakaryocytes, and platelets. PD-1 (programmed death receptor 1) inhibition prolonged survival in human MPN xenograft and primary murine MPN models. This effect was dependent on T cells. Mechanistically, PD-L1 surface expression in JAK2V617F-mutant cells affected metabolism and cell cycle progression of T cells. In summary, we report that in MPN, constitutive JAK2/STAT3/STAT5 activation, mainly in monocytes, megakaryocytes, and platelets, caused PD-L1-mediated immune escape by reducing T cell activation, metabolic activity, and cell cycle progression. The susceptibility of JAK2V617F-mutant MPN to PD-1 targeting paves the way for immunomodulatory approaches relying on PD-1 inhibition.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

References
-
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;3:261–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

