Escape of Hepatitis C Virus from Epitope I Neutralization Increases Sensitivity of Other Neutralization Epitopes
- PMID: 29467319
- PMCID: PMC5899191
- DOI: 10.1128/JVI.02066-17
Escape of Hepatitis C Virus from Epitope I Neutralization Increases Sensitivity of Other Neutralization Epitopes
Abstract
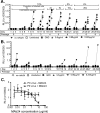
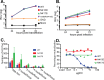
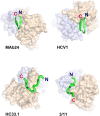
The hepatitis C virus (HCV) E2 glycoprotein is a major target of the neutralizing antibody (nAb) response, with multiple type-specific and broadly neutralizing antibody (bnAb) epitopes identified. The 412-to-423 region can generate bnAbs that block interaction with the cell surface receptor CD81, with activity toward multiple HCV genotypes. In this study, we reveal the structure of rodent monoclonal antibody 24 (MAb24) with an extensive contact area toward a peptide spanning the 412-to-423 region. The crystal structure of the MAb24-peptide 412-to-423 complex reveals the paratope bound to a peptide hairpin highly similar to that observed with human MAb HCV1 and rodent MAb AP33, but with a different angle of approach. In viral outgrowth experiments, we demonstrated three distinct genotype 2a viral populations that acquired resistance to MAb24 via N415D, N417S, and N415D/H386R mutations. Importantly, the MAb24-resistant viruses exhibited significant increases in sensitivity to the majority of bnAbs directed to epitopes within the 412-to-423 region and in additional antigenic determinants located within E2 and the E1E2 complex. This study suggests that modification of N415 causes a global change in glycoprotein structure that increases its vulnerability to neutralization by other antibodies. This finding suggests that in the context of an antibody response to viral infection, acquisition of escape mutations in the 412-to-423 region renders the virus more susceptible to neutralization by other specificities of nAbs, effectively reducing the immunological fitness of the virus. A vaccine for HCV that generates polyspecific humoral immunity with specificity for the 412-to-423 region and at least one other region of E2 is desirable.IMPORTANCE Understanding how antibodies neutralize hepatitis C virus (HCV) is essential for vaccine development. This study reveals for the first time that when HCV develops resistance to a major class of bnAbs targeting the 412-to-423 region of E2, this results in a concomitant increase in sensitivity to neutralization by a majority of other bnAb specificities. Vaccines for the prevention of HCV infection should therefore generate bnAbs directed toward the 412-to-423 region of E2 and additional bnAb epitopes within the viral glycoproteins.
Keywords: glycoproteins; hepatitis C virus; neutralizing antibodies; vaccines.
Copyright © 2018 Gu et al.
Figures


Similar articles
-
HCV Glycoprotein Structure and Implications for B-Cell Vaccine Development.Int J Mol Sci. 2020 Sep 16;21(18):6781. doi: 10.3390/ijms21186781. Int J Mol Sci. 2020. PMID: 32947858 Free PMC article. Review.
-
A Recombinant Hepatitis C Virus Genotype 1a E1/E2 Envelope Glycoprotein Vaccine Elicits Antibodies That Differentially Neutralize Closely Related 2a Strains through Interactions of the N-Terminal Hypervariable Region 1 of E2 with Scavenger Receptor B1.J Virol. 2019 Oct 29;93(22):e00810-19. doi: 10.1128/JVI.00810-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462563 Free PMC article.
-
Structure-Based Design of Hepatitis C Virus E2 Glycoprotein Improves Serum Binding and Cross-Neutralization.J Virol. 2020 Oct 27;94(22):e00704-20. doi: 10.1128/JVI.00704-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32878891 Free PMC article.
-
Mutations in hepatitis C virus E2 located outside the CD81 binding sites lead to escape from broadly neutralizing antibodies but compromise virus infectivity.J Virol. 2009 Jun;83(12):6149-60. doi: 10.1128/JVI.00248-09. Epub 2009 Mar 25. J Virol. 2009. PMID: 19321602 Free PMC article.
-
Mapping Determinants of Virus Neutralization and Viral Escape for Rational Design of a Hepatitis C Virus Vaccine.Front Immunol. 2018 May 31;9:1194. doi: 10.3389/fimmu.2018.01194. eCollection 2018. Front Immunol. 2018. PMID: 29904384 Free PMC article. Review.
Cited by
-
Epitope-focused immunogens targeting the hepatitis C virus glycoproteins induce broadly neutralizing antibodies.Sci Adv. 2024 Dec 6;10(49):eado2600. doi: 10.1126/sciadv.ado2600. Epub 2024 Dec 6. Sci Adv. 2024. PMID: 39642219 Free PMC article.
-
From Structural Studies to HCV Vaccine Design.Viruses. 2021 May 4;13(5):833. doi: 10.3390/v13050833. Viruses. 2021. PMID: 34064532 Free PMC article. Review.
-
To Include or Occlude: Rational Engineering of HCV Vaccines for Humoral Immunity.Viruses. 2021 Apr 30;13(5):805. doi: 10.3390/v13050805. Viruses. 2021. PMID: 33946211 Free PMC article. Review.
-
Mechanisms of Hepatitis C Virus Escape from Vaccine-Relevant Neutralizing Antibodies.Vaccines (Basel). 2021 Mar 20;9(3):291. doi: 10.3390/vaccines9030291. Vaccines (Basel). 2021. PMID: 33804732 Free PMC article. Review.
-
HCV Glycoprotein Structure and Implications for B-Cell Vaccine Development.Int J Mol Sci. 2020 Sep 16;21(18):6781. doi: 10.3390/ijms21186781. Int J Mol Sci. 2020. PMID: 32947858 Free PMC article. Review.
References
-
- Ribeiro RM, Li H, Wang S, Stoddard MB, Learn GH, Korber BT, Bhattacharya T, Guedj J, Parrish EH, Hahn BH, Shaw GM, Perelson AS. 2012. Quantifying the diversification of hepatitis C virus (HCV) during primary infection: estimates of the in vivo mutation rate. PLoS Pathog 8:e1002881. doi:10.1371/journal.ppat.1002881. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

