Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12
- PMID: 29467322
- PMCID: PMC5916241
- DOI: 10.1172/jci.insight.94952
Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12
Abstract
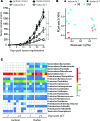
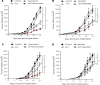
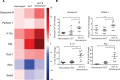
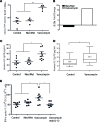
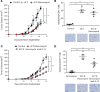
Adoptive T cell therapy (ACT) is a promising new modality for malignancies. Here, we report that adoptive T cell efficacy in tumor-bearing mice is significantly affected by differences in the native composition of the gut microbiome or treatment with antibiotics, or by heterologous fecal transfer. Depletion of bacteria with vancomycin decreased the rate of tumor growth in mice from The Jackson Laboratory receiving ACT, whereas treatment with neomycin and metronidazole had no effect, indicating the role of specific bacteria in host response. Vancomycin treatment induced an increase in systemic CD8α+ DCs, which sustained systemic adoptively transferred antitumor T cells in an IL-12-dependent manner. In subjects undergoing allogeneic hematopoietic cell transplantation, we found that oral vancomycin also increased IL-12 levels. Collectively, our findings demonstrate an important role played by the gut microbiota in the antitumor effectiveness of ACT and suggest potentially new avenues to improve response to ACT by altering the gut microbiota.
Keywords: Cancer immunotherapy; Immunology; Microbiology.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

