Lipid abnormalities in atopic skin are driven by type 2 cytokines
- PMID: 29467325
- PMCID: PMC5916244
- DOI: 10.1172/jci.insight.98006
Lipid abnormalities in atopic skin are driven by type 2 cytokines
Abstract
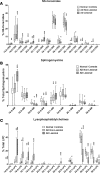
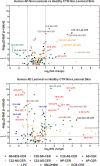
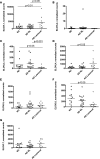
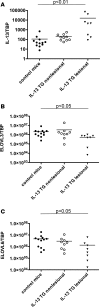
Lipids in the stratum corneum of atopic dermatitis (AD) patients differ substantially in composition from healthy subjects. We hypothesized that hyperactivated type 2 immune response alters AD skin lipid metabolism. We have analyzed stratum corneum lipids from nonlesional and lesional skin of AD subjects and IL-13 skin-specific Tg mice. We also directly examined the effects of IL-4/IL-13 on human keratinocytes in vitro. Mass spectrometric analysis of lesional stratum corneum from AD subjects and IL-13 Tg mice revealed an increased proportion of short-chain (N-14:0 to N-24:0) NS ceramides, sphingomyelins, and 14:0-22:0 lysophosphatidylcholines (14:0-22:0 LPC) with a simultaneous decline in the proportion of corresponding long-chain species (N-26:0 to N-32:0 sphingolipids and 24:0-30:0 LPC) when compared with healthy controls. An increase in short-chain LPC species was also observed in nonlesional AD skin. Similar changes were observed in IL-4/IL-13-driven responses in Ca2+-differentiated human keratinocytes in vitro, all being blocked by STAT6 silencing with siRNA. RNA sequencing analysis performed on stratum corneum of AD as compared with healthy subjects identified decreased expression of fatty acid elongases ELOVL3 and ELOVL6 that contributed to observed changes in atopic skin lipids. IL-4/IL-13 also inhibited ELOVL3 and ELOVL6 expression in keratinocyte cultures in a STAT6-dependent manner. Downregulation of ELOVL3/ELOVL6 expression in keratinocytes by siRNA decreased the proportion of long-chain fatty acids globally and in sphingolipids. Thus, our data strongly support the pathogenic role of type 2 immune activation in AD skin lipid metabolism.
Keywords: Dermatology; Skin.
Conflict of interest statement
Figures


References
-
- Bieber T, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J Allergy Clin Immunol. 2017;139(4S):S58–S64. - PubMed
-
- Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: A comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49–S57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

