Inducible podocyte-specific deletion of CTCF drives progressive kidney disease and bone abnormalities
- PMID: 29467330
- PMCID: PMC5916242
- DOI: 10.1172/jci.insight.95091
Inducible podocyte-specific deletion of CTCF drives progressive kidney disease and bone abnormalities
Abstract
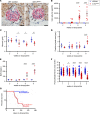
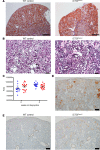
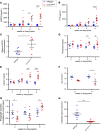
Progressive chronic kidney diseases (CKDs) are on the rise worldwide. However, the sequence of events resulting in CKD progression remain poorly understood. Animal models of CKD exploring these issues are confounded by systemic toxicities or surgical interventions to acutely induce kidney injury. Here we report the generation of a CKD mouse model through the inducible podocyte-specific ablation of an essential endogenous molecule, the chromatin structure regulator CCCTC-binding factor (CTCF), which leads to rapid podocyte loss (iCTCFpod-/-). As a consequence, iCTCFpod-/- mice develop severe progressive albuminuria, hyperlipidemia, hypoalbuminemia, and impairment of renal function, and die within 8-10 weeks. CKD progression in iCTCFpod-/- mice leads to high serum phosphate and elevations in fibroblast growth factor 23 (FGF23) and parathyroid hormone that rapidly cause bone mineralization defects, increased bone resorption, and bone loss. Dissection of the timeline leading to glomerular pathology in this CKD model led to the surprising observation that podocyte ablation and the resulting glomerular filter destruction is sufficient to drive progressive CKD and osteodystrophy in the absence of interstitial fibrosis. This work introduces an animal model with significant advantages for the study of CKD progression, and it highlights the need for podocyte-protective strategies for future kidney therapeutics.
Keywords: Chronic kidney disease; Endocrinology; Nephrology.
Conflict of interest statement
Figures



References
-
- GBD 2015 Mortality Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK057683/DK/NIDDK NIH HHS/United States
- K08 DK093783/DK/NIDDK NIH HHS/United States
- F31 CA195701/CA/NCI NIH HHS/United States
- R01 DK095045/DK/NIDDK NIH HHS/United States
- R01 DK099465/DK/NIDDK NIH HHS/United States
- R01 DK046718/DK/NIDDK NIH HHS/United States
- R03 DK107918/DK/NIDDK NIH HHS/United States
- R01 DK091218/DK/NIDDK NIH HHS/United States
- R56 DK046718/DK/NIDDK NIH HHS/United States
- K08 DK083511/DK/NIDDK NIH HHS/United States
- R01 DK062472/DK/NIDDK NIH HHS/United States
- R56 DK093746/DK/NIDDK NIH HHS/United States
- R37 DK046718/DK/NIDDK NIH HHS/United States
- P30 AR066261/AR/NIAMS NIH HHS/United States
- K08 DK093608/DK/NIDDK NIH HHS/United States
- P01 DK011794/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

