Apolipoproteins E and CIII interact to regulate HDL metabolism and coronary heart disease risk
- PMID: 29467335
- PMCID: PMC5916256
- DOI: 10.1172/jci.insight.98045
Apolipoproteins E and CIII interact to regulate HDL metabolism and coronary heart disease risk
Abstract
Background: Subspecies of HDL contain apolipoprotein E (apoE) and/or apoCIII. Both proteins have properties that could affect HDL metabolism. The relation between HDL metabolism and risk of coronary heart disease (CHD) is not well understood.
Methods: Eighteen participants were given a bolus infusion of [D3]L-leucine to label endogenous proteins on HDL. HDL was separated into subspecies containing apoE and/or apoCIII and then into 4 sizes. Metabolic rates for apoA-I in HDL subspecies and sizes were determined by interactive modeling. The concentrations of apoE in HDL that contain or lack apoCIII were measured in a prospective study in Denmark including 1,949 incident CHD cases during 9 years.
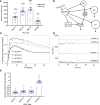
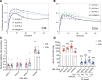
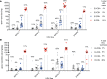
Results: HDL containing apoE but not apoCIII is disproportionately secreted into the circulation, actively expands while circulating, and is quickly cleared. These are key metabolic steps in reverse cholesterol transport, which may protect against atherosclerosis. ApoCIII on HDL strongly attenuates these metabolic actions of HDL apoE. In the epidemiological study, the relation between HDL apoE concentration and CHD significantly differed depending on whether apoCIII was present. HDL apoE was associated significantly with lower risk of CHD only in the HDL subspecies lacking apoCIII.
Conclusions: ApoE and apoCIII on HDL interact to affect metabolism and CHD. ApoE promotes metabolic steps in reverse cholesterol transport and is associated with lower risk of CHD. ApoCIII, when coexisting with apoE on HDL, abolishes these benefits. Therefore, differences in metabolism of HDL subspecies pertaining to reverse cholesterol transport are reflected in differences in association with CHD.
Trial registration: Clinicaltrials.gov NCT01399632.
Funding: This work was supported by NIH grant R01HL095964 to FMS and by a grant to the Harvard Clinical and Translational Science Center (8UL1TR0001750) from the National Center for Advancing Translational Science.
Keywords: Cardiovascular disease; Epidemiology; Lipoproteins; Metabolism.
Conflict of interest statement
Figures






Similar articles
-
Dietary unsaturated fat increases HDL metabolic pathways involving apoE favorable to reverse cholesterol transport.JCI Insight. 2019 Apr 4;4(7):e124620. doi: 10.1172/jci.insight.124620. eCollection 2019 Apr 4. JCI Insight. 2019. PMID: 30944249 Free PMC article. Clinical Trial.
-
ABCA1 promotes the de novo biogenesis of apolipoprotein CIII-containing HDL particles in vivo and modulates the severity of apolipoprotein CIII-induced hypertriglyceridemia.Biochemistry. 2008 Sep 30;47(39):10491-502. doi: 10.1021/bi801249c. Epub 2008 Sep 4. Biochemistry. 2008. PMID: 18767813
-
Triglyceride-rich lipoprotein, remnant cholesterol, and apolipoproteins CII, CIII, and E in patients with schizophrenia.J Lipid Res. 2024 Jul;65(7):100577. doi: 10.1016/j.jlr.2024.100577. Epub 2024 Jun 13. J Lipid Res. 2024. PMID: 38879166 Free PMC article.
-
Dietary fat and carbohydrate affect the metabolism of protein-based high-density lipoprotein subspecies.Curr Opin Lipidol. 2022 Feb 1;33(1):1-15. doi: 10.1097/MOL.0000000000000809. Curr Opin Lipidol. 2022. PMID: 34907968 Free PMC article. Review.
-
Apolipoprotein CIII links dyslipidemia with atherosclerosis.J Atheroscler Thromb. 2009 Mar;16(1):6-11. doi: 10.5551/jat.e607. Epub 2009 Mar 5. J Atheroscler Thromb. 2009. PMID: 19262004 Review.
Cited by
-
Association of Apolipoprotein E in Lipoprotein Subspecies With Risk of Dementia.JAMA Netw Open. 2020 Jul 1;3(7):e209250. doi: 10.1001/jamanetworkopen.2020.9250. JAMA Netw Open. 2020. PMID: 32648923 Free PMC article.
-
Associations of Perfluoroalkyl substances with blood lipids and Apolipoproteins in lipoprotein subspecies: the POUNDS-lost study.Environ Health. 2020 Jan 13;19(1):5. doi: 10.1186/s12940-020-0561-8. Environ Health. 2020. PMID: 31931806 Free PMC article. Clinical Trial.
-
The distinct metabolism between large and small HDL indicates unique origins of human apolipoprotein A4.JCI Insight. 2023 Apr 24;8(8):e162481. doi: 10.1172/jci.insight.162481. JCI Insight. 2023. PMID: 37092549 Free PMC article.
-
Alterations of HDL's to piHDL's Proteome in Patients with Chronic Inflammatory Diseases, and HDL-Targeted Therapies.Pharmaceuticals (Basel). 2022 Oct 18;15(10):1278. doi: 10.3390/ph15101278. Pharmaceuticals (Basel). 2022. PMID: 36297390 Free PMC article. Review.
-
Exploring apolipoprotein C-III: pathophysiological and pharmacological relevance.Cardiovasc Res. 2024 Feb 17;119(18):2843-2857. doi: 10.1093/cvr/cvad177. Cardiovasc Res. 2024. PMID: 38039351 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

