Emerging views of the nucleus as a cellular mechanosensor
- PMID: 29467443
- PMCID: PMC6440800
- DOI: 10.1038/s41556-018-0038-y
Emerging views of the nucleus as a cellular mechanosensor
Abstract
The ability of cells to respond to mechanical forces is critical for numerous biological processes. Emerging evidence indicates that external mechanical forces trigger changes in nuclear envelope structure and composition, chromatin organization and gene expression. However, it remains unclear if these processes originate in the nucleus or are downstream of cytoplasmic signals. Here we discuss recent findings that support a direct role of the nucleus in cellular mechanosensing and highlight novel tools to study nuclear mechanotransduction.
Conflict of interest statement
Competing Financial Interests
The authors have no competing financial interests to declare.
Figures

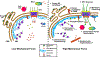

References
-
- Dewey CF Jr., Bussolari SR, Gimbrone MA Jr. & Davies PF The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103, 177–85 (1981). - PubMed
-
- Freeman PM, Natarajan RN, Kimura JH & Andriacchi TP Chondrocyte cells respond mechanically to compressive loads. J Orthop Res 12, 311–20 (1994). - PubMed
-
- Magid A & Law DJ Myofibrils bear most of the resting tension in frog skeletal muscle. Science 230, 1280–2 (1985). - PubMed
-
- Le HQ et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol 18, 864–75 (2016). - PubMed
-
- Lecuit T & Lenne PF Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol 8, 633–44 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

