Signalling from the periphery to the brain that regulates energy homeostasis
- PMID: 29467468
- PMCID: PMC9190118
- DOI: 10.1038/nrn.2018.8
Signalling from the periphery to the brain that regulates energy homeostasis
Abstract
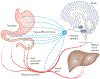
The CNS regulates body weight; however, we still lack a clear understanding of what drives decisions about when, how much and what to eat. A vast array of peripheral signals provides information to the CNS regarding fluctuations in energy status. The CNS then integrates this information to influence acute feeding behaviour and long-term energy homeostasis. Previous paradigms have delegated the control of long-term energy homeostasis to the hypothalamus and short-term changes in feeding behaviour to the hindbrain. However, recent studies have identified target hindbrain neurocircuitry that integrates the orchestration of individual bouts of ingestion with the long-term regulation of energy balance.
Figures

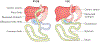

References
-
- Svendsen B et al. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 156, 847–857 (2015). - PubMed
-
- Batterham RL et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4, 223–233 (2006). - PubMed
-
- Scrocchi LA, Hill ME, Saleh J, Perkins B & Drucker DJ Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes 49, 1552–1560 (2000). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

