Phospho-Tau Bar Code: Analysis of Phosphoisotypes of Tau and Its Application to Tauopathy
- PMID: 29467609
- PMCID: PMC5808175
- DOI: 10.3389/fnins.2018.00044
Phospho-Tau Bar Code: Analysis of Phosphoisotypes of Tau and Its Application to Tauopathy
Abstract
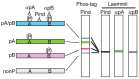
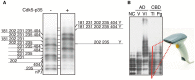
Tau is a microtubule-associated protein which regulates the assembly and stability of microtubules in the axons of neurons. Tau is also a major component of neurofibrillary tangles (NFTs), a pathological hallmark in Alzheimer's disease (AD). A characteristic of AD tau is hyperphosphorylation with more than 40 phosphorylation sites. Aggregates of hyperphosphorylated tau are also found in other neurodegenerative diseases which are collectively called tauopathies. Although a large number of studies have been performed on the phosphorylation of AD tau, it is not known if there is disease-specific phosphorylation among tauopathies. This is due to the lack of a proper method for analyzing tau phosphorylation in vivo. Most previous phosphorylation studies were conducted using a range of phosphorylation site-specific antibodies. These studies describe relative changes of different phosphorylation sites, however, it is hard to estimate total, absolute and collective changes in phosphorylation. To overcome these problems, we have recently applied the Phos-Tag technique to the analysis of tau phosphorylation in vitro and in vivo. This method separates tau into many bands during SDS-PAGE depending on its phosphorylation states, creating a bar code appearance. We propose calling this banding pattern of tau the "phospho-tau bar code." In this review article, we describe what is newly discovered regarding tau phosphorylation through the use of the Phos-Tag. We would like to propose its use for the postmortem diagnosis of tauopathy which is presently done by immunostaining diseased brains with anti-phospho-antibodies. While Phos-tag SDS-PAGE, like other biochemical assays, will lose morphological information, it could provide other types of valuable information such as disease-specific phosphorylation.
Keywords: Alzheimer' disease; Cdk5; GSK3β; phos-tag; phospho-tau bar code; phosphorylation; tau; tauopathy.
Figures


References
-
- Ando K., Maruko-Otake A., Ohtake Y., Hayashishita M., Sekiya M., Iijima K. M. (2016). Stabilization of Microtubule-Unbound Tau via Tau Phosphorylation at Ser262/356 by Par-1/MARK Contributes to Augmentation of AD-Related Phosphorylation and Aβ42-Induced Tau Toxicity. PLoS Genet. 2:e1005917 10.1371/journal.pgen.1005917 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

