Molecular Characterization of GABA-A Receptor Subunit Diversity within Major Peripheral Organs and Their Plasticity in Response to Early Life Psychosocial Stress
- PMID: 29467616
- PMCID: PMC5807923
- DOI: 10.3389/fnmol.2018.00018
Molecular Characterization of GABA-A Receptor Subunit Diversity within Major Peripheral Organs and Their Plasticity in Response to Early Life Psychosocial Stress
Abstract
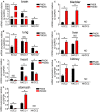
Gamma aminobutyric acid (GABA) subtype A receptors (GABAARs) are integral membrane ion channels composed of five individual proteins or subunits. Up to 19 different GABAAR subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π, and ρ1-3) have been identified, resulting in anatomically, physiologically, and pharmacologically distinct multiple receptor subtypes, and therefore GABA-mediated inhibition, across the central nervous system (CNS). Additionally, GABAAR-modulating drugs are important tools in clinical medicine, although their use is limited by adverse effects. While significant advances have been made in terms of characterizing the GABAAR system within the brain, relatively less is known about the molecular phenotypes within the peripheral nervous system of major organ systems. This represents a potentially missed therapeutic opportunity in terms of utilizing or repurposing clinically available GABAAR drugs, as well as promising research compounds discarded due to their poor CNS penetrance, for the treatment of peripheral disorders. In addition, a broader understanding of the peripheral GABAAR subtype repertoires will contribute to the design of therapies which minimize peripheral side-effects when treating CNS disorders. We have recently provided a high resolution molecular and function characterization of the GABAARs within the enteric nervous system of the mouse colon. In this study, the aim was to determine the constituent GABAAR subunit expression profiles of the mouse bladder, heart, liver, kidney, lung, and stomach, using reverse transcription polymerase chain reaction and western blotting with brain as control. The data indicate that while some subunits are expressed widely across various organs (α3-5), others are restricted to individual organs (γ2, only stomach). Furthermore, we demonstrate complex organ-specific developmental expression plasticity of the transporters which determine the chloride gradient within cells, and therefore whether GABAAR activation has a depolarizing or hyperpolarizing effect. Finally, we demonstrate that prior exposure to early life psychosocial stress induces significant changes in peripheral GABAAR subunit expression and chloride transporters, in an organ- and subunit-specific manner. Collectively, the data demonstrate the molecular diversity of the peripheral GABAAR system and how this changes dynamically in response to life experience. This provides a molecular platform for functional analyses of the GABA-GABAAR system in health, and in diseases affecting various peripheral organs.
Keywords: GABA; KCC2; NKCC1/2; chloride transporters; peripheral nervous system; psychosocial stress.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

